| Identification | Back Directory | [Name]
Stanozolol | [CAS]
10418-03-8 | [Synonyms]
Anabol
Estazol
Stromba
STANOZOL
STANAZOL
win14833
Winstrol
winstrolv
Winstroid
Tevabolin
Win 14833
NSC-43193
Stanozolo
Stanozlol
NSC 233046
Stanazolol
winstrol l
STANOZOLOL
Winstrol V
Strombaject
androstanazol
ANDROSTANAZOLE
Winstrol Depot
STANOZOLOL,USP
STANOZOLOL BP, USP
Stanozolol(winstrol)
WINSTROL (stanozolol)
Androstanazolestanazol
stanozolol--dea schedule
Stanozolol CIII (200 mg)
Stanozolol solution
stanozolol solution,100ppm
stanozolol--dea schedule iii
Stanozolol(Winstrol, Winstrol Depot)
Stanozolol Suspension (For Injection)
17-Methyl-5a-androstano[3,2-c]pyrazol-17b-ol
yclopenta[7,8]-phenanthro[2,3-c]pyrazol-1-ol
17α-methyl-5α-androstan-17β-olo(3,2-c)pyrazole
17b-Hydroxy-17a-methylandrostano(3,2-c)pyrazole
5a-Androstane-17a-methyl-17b-ol-(3,2-c)pyrazole
5α-Androstane-17α-methyl-17β-ol-[3,2-c]pyrazole
17-Methyl-pyrazolo[4',3':2,3]-5a-androstan-17b-ol
17β-Hydroxy-17α-methylandrostano [3,2-c] pyrazole
17b-Hydroxy-17-methyl-5a-androstano[3,2-c]pyrazole
17a-Methyl-17b-hydroxy-5a-androstano(3,2-c)pyrazole
17b-Hydroxy-17a-methyl-5a-androstano[3,2-c]pyrazole
17β-Hydroxy-17α-methyl-5α-androstano[3,2-c]pyrazole
Stanozolol BP/USP (Winstrol)
17BETA-HYDROXY-17ALPHA-METHYLANDROSTANO[3,2-C]PYRAZOLE
17beta-hydroxy-17-methyl-5alpha-androstano[3,2-c]pyrazole
17ALPHA-METHYL-5ALPHA-ANDROSTAN-17BETA-OLO[3,2-C]PYRAZOLE
17-methyl-2h-5alpha-androst-2-eno[3,2-c]pyrazol-17beta-ol
2-c)pyrazol-17beta-ol,17-methyl-2’h-5alpha-androst-2-eno(
17-Methyl-2'H-5alpha-androst-2-eno(3,2-c)pyrazol-17beta-ol
5-ALPHA-ANDROSTAN-17-ALPHAMETHYL-17-BETAOL-[3,2-C]PYRAZOLE
17-alpha-methyl-5-alpha-androstano(3,2-c)pyrazol-17-beta-ol
2'H-Androst-2-eno[3,2-c]pyrazol-17-ol,17-Methyl-, (5a,17b)-
2'H-5a-Androst-2-eno[3,2-c]pyrazol-17b-ol, 17-methyl-(8CI)
2'H-5alpha-Androst-2-eno(3,2-c)pyrazol-17beta-ol, 17-methyl-
2-c)pyrazol-17-ol,17-methyl-,(5alpha,17beta)-2’h-androst-2-eno(
2'H-Androst-2-eno[3,2-c]pyrazol-17-ol, 17-methyl-, (5a,17b)-(9CI)
2'H-Androst-2-eno[3,2-c]pyrazol-17-ol, 17-methyl-, (5alpha,17beta)-
1,2,3,3a,3b,4,5,5a,6,8,10,10a,10b,11,12,12a-hexadecahydro-1,10a,12a-trimethylc
Cyclopenta[7,8]phenanthro[2,3-c]pyrazole, 2'H-androst-2-eno[3,2-c]pyrazol-17-ol deriv.
1,10a,12a-Trimethyl-1,2,3,3a,3b,4,5,5a,6,8,10,10a,10b,11,12,12a-hexadecahydrocyclopenta[5,6]naphtho[1,2-f]indazol-1-ol
Cyclopenta[7,8]phenanthro-[2,3-c]pyrazol-1-ol, 1, 2,3,3a,3b,4,5,5a,6,8,10,10a,10b,11,12,12a-hexadecahydro-1,10a, 12a-trimethyl-
5α-Androstane-17α-methyl-17β-ol-[3,2-c]pyrazole, Androstanazole, Stanazol, 17β-Hydroxy-17α-methyl-5α-androstano[3,2-c]pyrazole
Cyclopenta[7,8]phenanthro[2,3-c]pyrazol-1-ol, 1,2,3,3a,3b,4,5,5a,6,7,10,10a,10b,11,12,12a-hexadecahydro-1,10a,12a-trimethyl-(6CI, 7CI) | [EINECS(EC#)]
233-894-8 | [Molecular Formula]
C21H32N2O | [MDL Number]
MFCD00133084 | [MOL File]
10418-03-8.mol | [Molecular Weight]
328.49 |
| Chemical Properties | Back Directory | [Melting point ]
242 °C | [alpha ]
34 º | [Boiling point ]
490.8±45.0 °C(Predicted) | [density ]
1.129±0.06 g/cm3(Predicted) | [refractive index ]
34 ° (C=0.4, CHCl3) | [Fp ]
-2℃ | [storage temp. ]
2-8°C
| [solubility ]
Practically insoluble in water, soluble in dimethylformamide, slightly soluble in ethanol (96 per cent), very slightly soluble in methylene chloride. | [form ]
powder
| [pka]
15.15±0.60(Predicted) | [color ]
white to light yellow
| [Merck ]
8794 | [InChIKey]
LKAJKIOFIWVMDJ-IYRCEVNGSA-N | [CAS DataBase Reference]
10418-03-8 | [NIST Chemistry Reference]
Stanozolol(10418-03-8) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Usage]
Anabolic steroid. Androgen.
Controlled substance. | [Usage]
androgen
anabolic steroid | [Uses]
Anabolic steroid. Androgen.
Controlled substance. | [Uses]
androgen
anabolic steroid | [Definition]
ChEBI: An organic heteropentacyclic compound resulting from the formal condensation of the 3-keto-aldehyde moiety of oxymetholone with hydrazine. Like oxymetholone, it is a synthetic anabolic steroid. It has both anabolic and androgenic properties, and has been u
ed to treat hereditary angioedema and various vascular disorders. It has also been widely abused by professional athletes. | [Originator]
Winstrol,Winthrop,US,1961 | [Manufacturing Process]
To a stirred solution of 1.00 gram of 17β-hydroxy-17α-methyl-4-
androsteno[3,2-c]pyrazole in 200 ml of tetrahydrofuran and 400 ml of liquid
ammonia was added 2.12 grams of lithium wire during 5 minutes. The dark
blue mixture was stirred for 45 minutes. A solution of 40 ml of tertiary-butyl
alcohol in 160 ml of diethyl ether was added with stirring.
After 15 minutes, 25 ml of ethanol was added with stirring. The mixture
turned colorless after several hours, and the liquid ammonia was allowed to
evaporate and the mixture was allowed to warm to room temperature over a
period of about 15 hours.
The solvent was evaporated to yield a colorless solid residue, which was taken
up in ethyl acetate-ice water. The two layers were separated and the aqueous
layer was extracted with ethyl acetate. The combined organic layers were
washed with water, saturated sodium chloride solution and filtered through
anhydrous sodium sulfate. The solvent was evaporated to yield 1.20 grams of
light tan crystals, MP 151° to 155°C, ultraviolet maximum at 224 mμ (E =
4,095). Two recrystallizations from ethanol afforded: 1st crop, 0.619 grams
(62%) of colorless crystals (dried at 120°C in vacuo for 17 hours), MP 232.8°
to 238.0°C, ultraviolet maximum at 224 mμ (E = 4,840); 2nd crop, 0.142
gram (14%) of colorless crystals, MP 234° to 242°C. | [Brand name]
Winstrol (Ovation). | [Therapeutic Function]
Anabolic | [Synthesis]
Stanozol, 17|á-methyl-5|á-androstano[3,2-c]pyrazol-17|?-ol (29.3.13), is made
by reducing the double bond at C4¨CC5 in methyltestosterone, which has independent inter�est as an anabolic drug of mestanolone (29.3.11). Mestanolone undergoes formylation
with ethylformate in the presence of sodium ethoxide, forming a 2-formyl (oxymethylene)
derivative (29.3.12), which upon reaction with hydrazine easily cyclizes to the desired
stanazole (29.3.13), which is a pyrazol-condensed steroid system.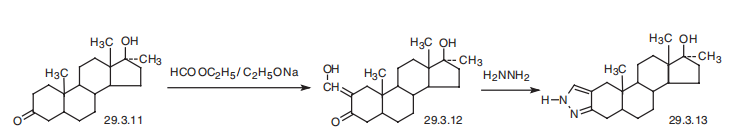
| [Veterinary Drugs and Treatments]
Labeled indications for the previously marketed veterinary stanozolol
product Winstrol?-V (Winthrop/Upjohn) included “…to
improve appetite, promote weight gain, and increase strength and
vitality…” in dogs, cats and horses. The manufacturer also stated
that: “Anabolic therapy is intended primarily as an adjunct to other
specific and supportive therapy, including nutritional therapy.”
Like nandrolone, stanozolol has been used to treat anemia of
chronic disease. Because stanozolol has been demonstrated to enhance
fibrinolysis after parenteral injection, it may be efficacious
in the treatment of feline aortic thromboembolism or thrombosis
in nephrotic syndrome; however, clinical studies and/or experience
are apparently lacking for this indication at present. |
| Questions and Answers (Q&A) | Back Directory | [Description]
Stanozolol is a synthetic anabolic-androgenic steroids (AAS) belonging to the dihydrotestosterone group. It can be used for the treatment of wasting diseases, burn victims, osteroporosis, bone fractures, anemia and even obesity. It can stimulate fat loss without causing reduced lean body mass, inducing hemoglobin production and red blood cell formation. Stanozolol has the following physiological functions: (1) reduction of sex-hormone-binding-globulin; (2) inducing protein synthesis; (3) Maintain nitrogen retention; (4) Increase red blood cells; (5) inhibit glucocorticoids.
| [References]
https://www.steroid.com/Stanozolol.php
https://pubchem.ncbi.nlm.nih.gov/compound/stanozolol
https://en.wikipedia.org/wiki/Stanozolol
|
|
|