| Identification | Back Directory | [Name]
BENDROFLUMETHIAZIDE | [CAS]
73-48-3 | [Synonyms]
ft8
ft81
bhft
relan
urlea
nikion
orsile
pluryl
blh368
centyl
intolex
livesan
aprinox
be724-a
benuron
pluryle
repicin
salural
salures
plusuril
poliuron
niagaril
bentride
naturine
flumesil
berkozide
bristuric
bristuron
neonaclex
nateretin
naturetin
sinesalin
relanbeta
sodiuretic
thiazidico
neo-naclex
neo-rontyl
benzylrodiuran
bendrofluazide
mide1,1-dioxide
NDROFLUMETHIAZIDE
bendroflumethazide
bendroflumethiazid
BENDROFLUMETHIAZIDE
benzydroflumethiazide
benzhydroflumethiazide
BENDROFLUMETHIAZIDE-D5
rac Bendroflumethiazide
Bendroflumethiazide CRS
oromethyl)-,1,1-dioxide
benzylhydroflumethiazide
Bendroflumethiazide (200 mg)
BENDROFLUMETHIAZIDE USP/EP/BP
BENDROFLUMETHIAZIDE, EP/BP/USP/JPC
BENDROFLUMETHIAZIDE(BENDROFLUAZIDE)
Bendroflumethiazide (200 mg)H0C4020.994mg/mg(ai)
3-benzyl-6-trifluoromethyl-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine,1
3-benzyl-3,4-dihydro-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfona
6-trifluoromethyl-3-benzyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine,1,1
2h-1,2,4-benzothiadiazine-7-sulfonamide,3,4-dihydro-3-(phenylmethyl)-6-(triflu
2h-1,2,4-benzothiadiazine-7-sulfonamide,3-benzyl-3,4-dihydro-6-(trifluoromethy
3-Benzyl-3,4-dihydro-6-trifluoromethyl-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dione
3-BENZYL-6-TRIFLUOROMETHYL-7-SULFAMOYL-3,4-DIHYDRO-2H-1,2,4-BENZOTHIADIAZINE 1,1-DIOXIDE
3-benzyl-3,4-dihydro-6-trifluoromethyl-1,2,4-benzothiadiazine-7-sulphonamide 1,1-dioxide
3-Benzyl-3,4-dihydro-6-trifluoromethyl-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
3-Benzyl-3,4-dihydro-6-(trifluoromethy)-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
3-benzyl-6-(trifluoroMethyl)-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonaMide 1,1-dioxide
3-(benzyl)-1,1-diketo-6-(trifluoromethyl)-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide
3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-
2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide
3,4-Dihydro-3-(phenylMethyl)-6-(trifluoroMethyl)-2H-1,2,4-benzothiadiazine-7-sulfonaMide 1,1-Dioxide
1,1-dioxo-3-(phenylmethyl)-6-(trifluoromethyl)-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide
2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-, 1,1-dioxide | [EINECS(EC#)]
200-800-1 | [Molecular Formula]
C15H14F3N3O4S2 | [MDL Number]
MFCD00078963 | [MOL File]
73-48-3.mol | [Molecular Weight]
421.41 |
| Hazard Information | Back Directory | [Description]
Bendroflumethiazide (Item No. 21311) is an analytical reference standard categorized as a diuretic.1,2 Diuretics, including bendroflumethiazide, have been abused as performance-enhancing drugs and masking agents in sports doping.3 This product is intended for research and forensic applications. | [Chemical Properties]
White Solid | [Originator]
Naturetin,Squibb,US,1959 | [Uses]
Diuretic; antihypertensive. | [Uses]
expectorant | [Definition]
ChEBI: A sulfonamide consisting of 7-sulfamoyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide in which the hydrogen at position 6 is substituted by a trifluoromethyl group and that at position 3 is substituted by a benzyl group. | [Manufacturing Process]
The process is described in US Patent 3,392,168 as follows:
(A) Preparation of 5-Trifluoromethylaniline-2,4-Disulfonylchloride - 113 ml of
chlorosulfonic acid is cooled in an ice bath, and to the acid is added dropwise
while stirring 26.6 grams of α,α,α-trifluoro-m-toluidine. 105 grams of sodium
chloride is added during 1-2 hours, where after the temperature of the
reaction mixture is raised slowly to 150° - 160°C which temperature is
maintained for three hours. After cooling the mixture, ice-cooled water is
added, whereby 5-trifluoromethylaniline-2,4-disulfonyl chloride separates out
from the mixture.
(B) Preparation of 5-Trifluoromethyl-2,4-Disulfamylaniline - The 5-
trifluoromethylaniline-2,4-disulfonyl chloride obtained in step (A) is taken up
in ether and the ether solution dried with magnesium sulfate. The ether is
removed from the solution by distillation, the residue is cooled to 0°, and 60
ml of ice-cooled, concentrated ammonia water is added while stirring. The
solution is then heated for one hour on a steam bath and evaporated in vacuo
to crystallization. The crystallized product is 5-trifluoromethyl-2,4-
disulfamylaniline, which is filtered off, washed with water and dried in a
vacuum-desiccator over phosphorus pentoxide. After recrystallization from a
mixture of 30% ethanol and 70% water, the compound has a MP of 247°-
248°C.
(C) Preparation of 3-Benzyl-6-Trifluoromethyl-7-Sulfarnyl-3,4-Dihydro-1,2,4-
Benzothiadiazine-1,1-Dioxide - 6.4 grams of 5-trifluoromethyl-2,4-
disulfamylaniline is dissolved in 12 ml of dioxane, 2.7 ml of
phenylacetaldehyde and a catalytic amount of p-toluenesulfonic acid are
added. After boiling for a short time under reflux, the reaction mixture
crystallizes, and, after filtration and recrystallization from dioxane, the desired product is obtained with a MP of 224.5°-225.5°C.
(D) Alternative to (C) - 9.6 grams of 5-trifluoromethyl-2,4-disulfarnylaniline
and 4.9 grams of ω-ethoxystyrene are dissolved in 35 ml of n-butanol. 0.5
grams of p-toluenesulfonic acid is added, and the mixture is heated on a
steam bath while stirring. When the solution is clear, 55 ml of hexane is
added, whereafter the mixture is heated further for one and a half hours.
After cooling, the substance identical to that of Example (C) is filtered off and
has a MP of 222°-223°C.
Sterile compositions containing Bendroflumethiazide for parenteral
administration may be prepared as described in US Patent 3,265,573. | [Brand name]
Naturetin
(Apothecon). | [Therapeutic Function]
Diuretic, Antihypertensive | [Clinical Use]
Thiazide diuretic:
Hypertension
Oedema | [Safety Profile]
Poison by intravenous route.Human systemic effects by ingestion: convulsions andsomnolence. Mutation data reported. When heated todecomposition it emits toxic fumes of F-, SOx, and NOx. | [Synthesis]
Bendroflumethiazide, 1,1-dioxide 3-benzyl-6-(trifluoromethyl)-
3,4-dihydro-2H-1,2,4-benzothiadiazin-7-sulfonamide (21.3.6), is synthesized by the same scheme of making the aforementioned drugs using phenylacetaldehyde or its acetale as a
carbonyl component, and using 2,4-disulfonamido-5-trifluoromethylaniline (21.3.5) as an
o-aminosulfonamide component.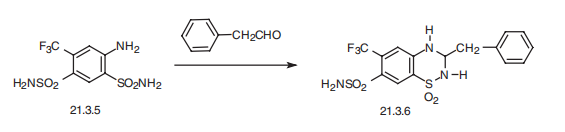
| [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: increased risk of nephrotoxicity with
NSAIDs; antagonism of diuretic effect.
Anti-arrhythmics: hypokalaemia leads to increased
cardiac toxicity; effects of lidocaine and mexiletine
antagonised.
Antibacterials: avoid administration with
lymecycline.
Antidepressants: increased risk of hypokalaemia
with reboxetine; enhanced hypotensive effect with
MAOIs; increased risk of postural hypotension with
tricyclics.
Antiepileptics: increased risk of hyponatraemia with
carbamazepine.
Antifungals: increased risk of hypokalaemia with
amphotericin.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotension with post�synaptic alpha-blockers like prazosin; hypokalaemia
increases risk of ventricular arrhythmias with sotalol.
Antipsychotics: hypokalaemia increases risk
of ventricular arrhythmias with amisulpride;
enhanced hypotensive effect with phenothiazines;
hypokalaemia increases risk of ventricular
arrhythmias with pimozide - avoid.
Atomoxetine: hypokalaemia increases risk of
ventricular arrhythmias.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: increased risk of nephrotoxicity and
hypomagnesaemia.
Cytotoxics: increased risk of ventricular arrhythmias
due to hypokalaemia with arsenic trioxide; increased
risk of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium excretion reduced, increased toxicity. | [Metabolism]
There are indications that bendroflumethiazide is fairly
extensively metabolised.
About 30% is excreted unchanged in the urine with the
remainder excreted as uncharacterised metabolites. |
|
|