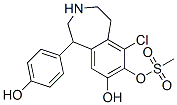
Fenoldopam mesylate
- CAS No.
- 67227-57-0
- Chemical Name:
- Fenoldopam mesylate
- Synonyms
- SKF R-82526;9CL-CORLOPAM;(S)-SKF-82526;(R)-SKF-82526;CORLOPAM MESYLATE;Fenodopam mesylate;SKF-82526 mesylate;FENOLDOPAM MESYLATE;FENOLDIPAM MESYLATE;FENOLDOPAM METHANESULFONATE
- CBNumber:
- CB3729841
- Molecular Formula:
- C17H20ClNO6S
- Molecular Weight:
- 401.86
- MDL Number:
- MFCD04112986
- MOL File:
- 67227-57-0.mol
- MSDS File:
- SDS
| Melting point | 274° (dec) |
|---|---|
| storage temp. | room temp |
| solubility | DMSO: ≥15mg/mL at ~60°C |
| form | powder |
| color | white to tan |
| InChIKey | WOFIIEUMIDZJEJ-UHFFFAOYSA-N |
| CAS DataBase Reference | 67227-57-0(CAS DataBase Reference) |
| FDA UNII | HA3R0MY016 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317-H319 | |||||||||
| Precautionary statements | P280-P301+P312+P330-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36-42/43 | |||||||||
| Safety Statements | 22-26-36/37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 2933995300 | |||||||||
| NFPA 704 |
|
Fenoldopam mesylate price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0198 | Fenoldopam mesylate ≥98% (HPLC) | 67227-57-0 | 10mg | $127 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1269458 | Fenoldopam mesylate United States Pharmacopeia (USP) Reference Standard | 67227-57-0 | 200mg | $781 | 2024-03-01 | Buy |
| Cayman Chemical | 17629 | Fenoldopam (mesylate) ≥95% | 67227-57-0 | 5mg | $48 | 2024-03-01 | Buy |
| Cayman Chemical | 17629 | Fenoldopam (mesylate) ≥95% | 67227-57-0 | 10mg | $89 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0198 | Fenoldopam mesylate ≥98% (HPLC) | 67227-57-0 | 50mg | $491 | 2024-03-01 | Buy |
Fenoldopam mesylate Chemical Properties,Uses,Production
Description
Fenoldopam, first approved in the Netherlands in 1992, ended by reaching its first market, the US, for the short-term management of severe hypertension, including malignant hypertension, in the hospital setting. Fenoldopam can be prepared in 3 steps from the corresponding phenethylamine and aryloxiran, the pivotal step being the cyclisation in benzazepine in acidic medium. Fenoldopam is a potent dopamine D1 receptor agonist acting peripherally to produce systemic vasodilation.As it does not cross the bloodbrain barrier, it does not exert significant central dopaminergic activity. Fenoldopam also interacts significantly with 5HT1c and 5HT2 receptors. In comparative trials with the most common drug used for this condition in Europe, Fenoldopam was found to be appreciably more potent than nifedipine. Furthermore, Fenoldopam is fast acting and maintains a long-lasting antihypertensive effect.
Description
Fenoldopam is an agonist of dopamine D1A (D1R) and D1B (D5R) receptors (Kds = 17 and 11 nM, respectively). Fenoldopam is used to study the roles of these receptors, in cells and in vivo, and to alter hemodynamic properties, including hypertension, in animals.
Chemical Properties
White to Off-White Solid
Originator
SmithKline Beecham (US)
Uses
Fenoldopam mesylate may be used to study dopamine D1-mediated cell signaling.
Uses
antihypertensive, dopamine agonist
Uses
Dopamine D1-receptor agonist. Antihypertensive.
Definition
ChEBI: Fenoldopam mesylate is a benzazepine.
Manufacturing Process
2-Chloro-3,4-dimethoxyphenethylamine (1.0 g) was reacted with 0.70 g of pmethoxystyrene
oxide to give the hydroxyphenethylamine; m.p. 118.5-121°C.
This compound (2.16 g) was stirred at room temperature in 15 ml of
trifluoroacetic acid with 4 drops of conc. sulfuric acid. After purification over a
silica gel column with chloroform, 10% methanol/chloroform as eluates, was
obtained 6-chloro-7,8-dimethoxy-1-p-methoxyphenyl-2,3,4,5-tetrahydro-1H-
3-benzazepine (0.78 g), m.p. 143-145°C.
The trimethoxy product (0.87 g, 2.50 mmoles) in 25 ml of dry methylene
chloride was cooled in an ice-methanol bath and 12.5 ml (25.0 mmoles) of
boron tribromide in methylene chloride was added dropwise. After stirring for
4 hours, the mixture was cooled in an ice bath while methanol was carefully
added to give 0.37 g of 6-chloro-7,8-dihydroxy-1-p-hydroxyphenyl-2,3,4,5-
tetrahydro-1H-3-benzazepine hydrobromide, m.p. 215°C.
The base was regenerated from the hydrobromide salt using sodium carbonate solution in 85% yield. Treating the base with various acids gave the
following salts: dl-tartrate, fumarate, hydrochloride, sulfate, and the most
water soluble one, the methanesulfonate, m.p. 272°C.
brand name
Corlopam (Hospira).
Therapeutic Function
Antihypertensive
Biochem/physiol Actions
Fenoldopam mesylate (FM) is considered as an effective therapeutic agent to prevent the onset of postoperative AKI (PO-AKI). In healthy cats, FM can stimulate diuresis and be well-tolerated. This benzazepine-derivative is regional specific and does not cause cerebral vasodilation.
Fenoldopam mesylate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; | info@afinechem.com | China | 15377 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | alfa4@alfachem.cn | China | 12755 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
View Lastest Price from Fenoldopam mesylate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![8-Chloro-2-(4-hydroxyphenyl)-4-azabicyclo[5.4.0]undeca-7,9,11-triene-9,10-diol methanesulphonate pictures](https://img.chemicalbook.com/ProductImageEN/2018-12/Small/0d29a12c-2ca6-431a-b0b8-b91eca49647d.png) |
2019-07-06 | 8-Chloro-2-(4-hydroxyphenyl)-4-azabicyclo[5.4.0]undeca-7,9,11-triene-9,10-diol methanesulphonate
67227-57-0
|
US $2.00 / kg | 1kg | 99% | 100kg | Career Henan Chemical Co |
-
![8-Chloro-2-(4-hydroxyphenyl)-4-azabicyclo[5.4.0]undeca-7,9,11-triene-9,10-diol methanesulphonate pictures](https://img.chemicalbook.com/ProductImageEN/2018-12/Small/0d29a12c-2ca6-431a-b0b8-b91eca49647d.png)
- 8-Chloro-2-(4-hydroxyphenyl)-4-azabicyclo[5.4.0]undeca-7,9,11-triene-9,10-diol methanesulphonate
67227-57-0
- US $2.00 / kg
- 99%
- Career Henan Chemical Co