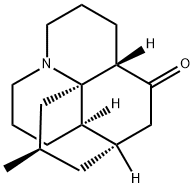
lycopodine
- CAS No.
- 466-61-5
- Chemical Name:
- lycopodine
- Synonyms
- lycopodine;(15R)-15-Methyllycopodan-5-one;1,9-Ethanobenzo[i]quinolizin-14-one, dodecahydro-11-methyl-, (1S,8aR,9S,11R,12aR)-
- CBNumber:
- CB41480614
- Molecular Formula:
- C16H25NO
- Molecular Weight:
- 247.38
- MDL Number:
- MFCD01632777
- MOL File:
- 466-61-5.mol
| Melting point | 114-115°; mp (racemate) 130-131° |
|---|---|
| alpha | D -24° (alc) |
| Boiling point | 125 °C(Press: 0.2 Torr) |
| Density | 1.18 g/cm3 |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 9.36±0.40(Predicted) |
| EWG's Food Scores | 1 |
| FDA UNII | 2P5MU968XW |
lycopodine Chemical Properties,Uses,Production
Description
This alkaloid has, so far, been discovered in all Lycopodium species with the
exception of L. saururus Lam. It was first obtained by B6deker who assigned to
it the formula C32Hs203N2, altered to that given by Achmatowicz and Uzieblo
and confirmed by Manske and Marion. It forms colourless prisms from Et20 and
has [α]20D - 9.01 ° (Me2CO). It yields crystalline salts and derivatives: the perchlorate, m.p. 276°C; methochloride, m.p. 238-240°C, methiodide, m.p. 335-
7°C and a hydrazone, mop. 20B-2l0°C. According to Bodeker it also yields a
crystalline hydrochloride and aurichloride.
Even under pressure at 200°C in the presence of Raney Ni, the base cannot be
hydrogenated. On selenium dehydrogenation, a complex mixture of bases is
formed from which 7 -methylquinoline and 5:7 -dimethylquinoline have been
identified. 7 -methylquinoline is also stated to be formed when the base is heated
with palladium as catalyst. A stereospecific total synthesis of the (±)-form has
been described.
Definition
ChEBI: Lycopodine is a quinolizidine alkaloid. It derives from a hydride of a lycopodane.
References
Bodeker., Annalen., 208,363 (1881)
Achmatowicz, Uzieblo., Rocz. Chern., 18,89 (1938)
Manske, Marion., Can. 1. Res., 20B, 87 (1942)
Marion, Manske., ibid, 20B, 153 (1942)
Manske, Marion., 1. Arner. Chern. Soc., 69,2126 (1947)
Anet., Tetrahedron Lett., 20, 13 (1960)
Harrison et al., Can. 1. Chern., 39,2086 (1961)
lycopodine Preparation Products And Raw materials
Raw materials
Preparation Products
lycopodine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 18892239720 | psaitong@jm-bio.com | China | 12308 | 58 |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 | liming@bio-fount.com | China | 9730 | 58 |
| cjbscvictory | 13348960310 13348960310 | 3003867561@qq.com | China | 9946 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 | psaitong@jm-bio.com | China | 29778 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24023 | 58 |
| Shanghai Yiyan Biotechnology Co. , Ltd. | 021-69985186 13611928337 | 3427709316@qq.com | China | 7984 | 58 |