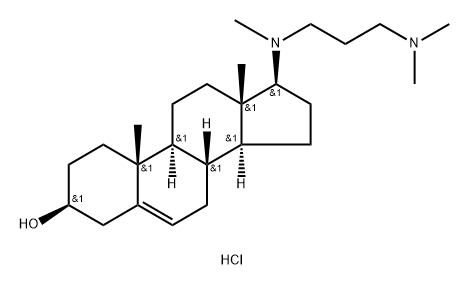
Azacosterol dihydrochloride
- CAS No.
- 1249-84-9
- Chemical Name:
- Azacosterol dihydrochloride
- Synonyms
- Azacosterol;Azocosterol;Azocosterol 2HCl;Androst-5-en-3-ol, 17-[[3-(dimethylamino)propyl]methylamino]-, hydrochloride (1:2), (3β,17β)-
- CBNumber:
- CB51178066
- Molecular Formula:
- C25H45ClN2O
- Molecular Weight:
- 425.1
- MDL Number:
- MOL File:
- 1249-84-9.mol
- MSDS File:
- SDS
| alpha | D -32° |
|---|---|
| FDA UNII | B32804UAUQ |
| EPA Substance Registry System | Azacosterol hydrochloride (1249-84-9) |
Azacosterol dihydrochloride price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Biorbyt Ltd | orb146177 | Azocosterol 2HCL >99% | 1249-84-9 | 100mg | $606.9 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb146177 | Azocosterol 2HCL >99% | 1249-84-9 | 250mg | $912.9 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb146177 | Azocosterol 2HCL >99% | 1249-84-9 | 1g | $1802 | 2021-12-16 | Buy |
| DC Chemicals | DC4141 | Azocosterol2HCL 99% | 1249-84-9 | 100mg | $300 | 2021-12-16 | Buy |
| DC Chemicals | DC4141 | Azocosterol2HCL 99% | 1249-84-9 | 250mg | $600 | 2021-12-16 | Buy |
Azacosterol dihydrochloride Chemical Properties,Uses,Production
Description
Azacosterol hydrochloride is a diaza derivative of cholesterol which acts as a hypocholesteremic agent by blocking delta-24-reductase causing accumulation of desmosterol.
Originator
Ornitrol,Avitrol Corporation
Uses
Chemosterilant, avian.
Manufacturing Process
A solution of 15 parts of 3β-hydroxyandrost-5-en-17-one and 30 parts of 3-
dimethylaminopropylamine in 36.6 parts of formic acid is heated in an oil bath
at about 170-180°C for about 24 hours. The cooled mixture is diluted with
about 500 parts of water, and the resulting aqueous mixture is extracted with
chloroform, containing a small amount of methanol. The organic layer is
separated, washed with water, dried over anhydrous sodium sulfate, and
evaporated to dryness under reduced pressure. The viscous residue is
dissolved in a mixture of 80 parts of isopropyl alcohol and 420 parts of ether,
and this solution is treated with isopropanolic hydrogen chloride. The resulting
precipitate is collected by filtration and washed with acetone to afford 17β-[N-
(3-dimethylaminopropyl)formamido-1-androst-5-en-3β-ol hydrochloride. A
solution of this hydrochloride in aqueous methanol is made alkaline by the
addition of dilute aqueous sodium hydroxide, and the resulting colloidal
precipitate is extracted with chloroform. The chloroform extracts washed with
water, dried over anhydrous sodium sulfate and concentrated to dryness to
afford a residue, which is crystallized from acetone, resulting in 17β-[N-(3-
dimethylaminopropyl)formamido]androst-5-en-3β-ol, which displays a double
melting point at about 116-118°C and 143-148°C, [α]D= -67.5° (chloroform).
To a slurry of 4 parts of LiAlH4 in 150 parts of dioxane is added dropwise with
stirring, at the reflux temperature a solution of 10 parts of 17β-[N-(3-
dimethylaminopropyl)
formamido]androst-5-en-3β-ol in 150 parts of dioxane. This reaction mixture
is heated at reflux for about 18 hours longer, and then treated dropwise
successively, at the reflux temperature, with a solution of 4 parts of water in
25 parts of dioxane, 3 parts of 20% aqueous sodium hydroxide, and 14 parts
of water. The resulting mixture is clarified by filtration, and the residue on the
filter is washed with fresh dioxane. The filtrates are combined, evaporated to
dryness under reduced pressure, and the resulting residue is recrystallized
from acetone-methanol to produce 17β-[N-methyl-N-(3-
dimethylaminopropyl)amino]androst-5-en-3β-ol, M.P. about 146-148°C, [α]D=
-54.5° (chloroform). A solution of this amine in ether-isopropyl alcohol is
treated with isopropanolic hydrogen chloride to afford Azacosterol
dihydrochloride, [α]D= -32° (methanol).
Therapeutic Function
Hypocholesteremic
Safety Profile
Poison by ingestion andintraperitoneal routes. Experimental reproductive effects.Mutation data reported. When heated to decomposition itemits toxic fumes of NOx and HCl.
Azacosterol dihydrochloride Preparation Products And Raw materials
Azacosterol dihydrochloride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | cdhxsj@163.com | China | 13359 | 58 |
| AN PharmaTech Co Ltd | 86(21)68097365 | sales@anpharma.net | China | 4901 | 55 |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | sales@xiyashiji.com | China | 20810 | 60 |
| Shandong Ono Chemical Co., Ltd. | 0539-6362799 20) | China | 9998 | 58 | |
| Aishilun biotechnology (Shanghai) co., LTD | 021-50676523 18019098996 | info@acelybio.com | China | 3967 | 58 |
| DC Chemicals | 021-58447131 13564518121 | sales@dcchemicals.com | China | 9414 | 58 |
| Absin Bioscience Inc. | 021-38015121 15000105423 | chenjw@absin.cn | China | 24734 | 58 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.com | China | 24246 | 58 |