|
|
| | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide Basic information | | Biochem/physiol Actions |
| Product Name: | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide | | Synonyms: | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide;4-[3-(3-fluorophenyl)-5,5-diMethyl-4-oxo-4,5-dihydrofuran-2-yl]benzene-1-sulfonaMide;Polmacoxib;5-{4-(aminosulfonyl)phenyl}-2,2-dimethyl-4-(3-fluorophenyl)-3(2H)-furanone;CG-100649;Benzenesulfonamide, 4-[3-(3-fluorophenyl)-4,5-dihydro-5,5-dimethyl-4-oxo-2-furanyl]-;NSAID,inflammatory,Carbonic Anhydrase,Polmacoxib,polyp,colorectal,adenoma,tumor,Inhibitor,COX,Cyclooxygenase,inhibit,Carbonate dehydratase,orthotopic;Calcium acetate Impurity 3 | | CAS: | 301692-76-2 | | MF: | C18H16FNO4S | | MW: | 361.39 | | EINECS: | | | Product Categories: | | | Mol File: | 301692-76-2.mol | 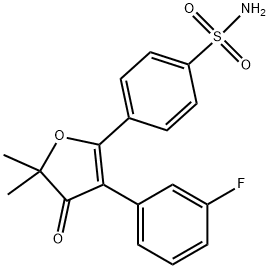 |
| | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide Chemical Properties |
| Melting point | 155-156 °C | | Boiling point | 527.7±60.0 °C(Predicted) | | density | 1.361±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2)(1:8): 0.5 mg/ml; Ethanol: 5 mg/ml | | form | A crystalline solid | | pka | 10.21±0.10(Predicted) |
| | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide Usage And Synthesis |
| Biochem/physiol Actions | Polmacoxib is a nonsteroidal anti-inflammatory drug (NSAID) that acts as an inhibitor of cyclooxygenase 2 (COX-2) and the carbonic anhydrases CAI and CAII. It has also been found to inhibit colorectal adenoma and tumor growth in mouse models. | | Description | Polmacoxib, also known as
(CG-100649), is a first-in-class NSAID which is a dual inhibitor
of COX-2 and carbonic anhydrase (CA). The drug, which
was approved in South Korea for the treatment of colorectal
cancer (CRC) in 2015 and whose discovery has been described
by workers at AmorePacific R&D, interacts with CA in red
blood cells, providing a novel “tissue-specific” transport
mechanism that is designed to deliver sustained levels of drug
to inflamed tissues while maintaining low systemic exposure.
Although the unique dual COX-2/CA inhibition is designed to
provide potentially superior safety to cardiovascular, renal, and
gastrointestinal tissues compared to traditional NSAIDs or
COX-2 inhibitor drugs, the long-term safety profile of the drug,
particularly cardiovascular risks notoriously associated with
inhibition of COX-2, has yet to be determined, and the drug
is currently not approved for use in any other country outside
of South Korea. | | Description | Polmacoxib is an inhibitor of cyclooxygenase 2 (COX-2) and the carbonic anhydrase subtypes I (CAI) and CAII. It inhibits COX-2 in the absence of carbonic anhydrase II with an IC50 value of 40 nM, which increases by approximately 4- and 17-fold in the presence of a CAII at a molar ratio of 1:1 and 1:5, respectively. It also inhibits CAI and CAII (IC50s = 210 and 95 nM, respectively). Polmacoxib prevents >95 and 90% of prostaglandin E2 (PGE2) production in HCA-7 and HT-29 human colon cancer cells, respectively, using concentrations of 0.01 and 0.001 μg/ml. It inhibits polyp formation in a transgenic mouse model of intestinal polyp formation and tumor growth in human colorectal carcinoma mouse xenograft models when used at a dose of 7 mg/kg. The inhibition of COX-2 and CAII by polmacoxib has the potential for fewer serious systemic adverse effects, including cardiovascular events associated with COX-2 selective inhibitors such as celecoxib . Formulations containing polmacoxib have been used in the treatment of osteoarthritis. | | Uses | Polmacoxib is a nonsteroidal anti-inflammatory drug that inhibits both cyclooxygenase-2 (COX-2) and carbonic anhydrase enzymes. | | Synthesis | Subjection of commercial propargyl alcohol 172 to nbutyllithium
at cryogenic temperatures followed by quenching
with commercial benzaldehyde 173 resulted in the formation of
benzyl alcohol 174 in 81% yield. This alcohol could be oxidized
by three different means, but the authors report that the most
suitable method on scale was through the use of manganese
dioxide in methylene chloride, which furnished ketone 175 in
80%. Next, an interesting cyclization reaction secured the
key furanone residue 176. Mechanistically, subjection of ynone
175 to dimethylamine likely resulted in a conjugate addition
followed by tautomerization of the resulting allenol to the
corresponding ketone. The resulting ketone then probably
underwent intramolecular nucleophilic attack by the pendant
tertiary alcohol and after ejection of a molecule of water
through iminium-mediated lone pair assistance, hydrolysis of
the iminium species to the corresponding ketone delivered 176.
Next, mCPBA was employed to oxidize sulfide 176 to the
corresponding sulfoxide. Subsequently, iodination of the
furanone through use of bis(trifluoroacetoxy)iodobenzene
(BTI), followed by a three-step sequence to convert the
methylsulfoxide to the corresponding primary sulfonamide 178
occurred in 41% overall from the four-step sequence. Finally,
Suzuki installation of the fluorobenzene resulted in the
completion of the synthesis of polmacoxib (XXII). 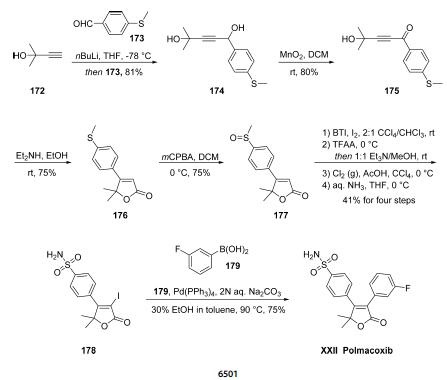
|
| | 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide Preparation Products And Raw materials |
|