
TITANIUM NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-11 |
Product Details
| Product Name: TITANIUM | CAS No.: 16962-40-6 |
| EC-No.: 241-036-9 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/11 |
| CAS: | 16962-40-6 |
| MF: | F6H4NTi- |
| MW: | 179.9 |
| EINECS: | 241-036-9 |
| Product Categories: | halometallate salts;AA Standard SolutionsAlphabetic;Ammonium SaltsChemical Synthesis;Reference/Calibration Standards;Standard Solutions;TF - TO;Industrial/Fine Chemicals;Catalysis and Inorganic Chemistry;Metal and Ceramic Science;Salts;Titanium |
| Mol File: | 16962-40-6.mol |
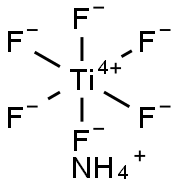 | |
| TITANIUM Chemical Properties |
| Melting point | 1660 °C(lit.) |
| Boiling point | 3287 °C(lit.) |
| density | 4.5 g/mL at 25 °C(lit.) |
| solubility | H2O: slightly soluble(lit.) |
| form | wire |
| color | White |
| PH | pH(50g/l, 25℃) : 2.0~4.0 |
| Water Solubility | Insoluble in water. |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| CAS DataBase Reference | 16962-40-6(CAS DataBase Reference) |
| EPA Substance Registry System | Titanate(2-), hexafluoro-, diammonium, (OC-6-11)- (16962-40-6) |
| TITANIUM Usage And Synthesis |
| Chemical Properties | white crystalline powder, crystals and/or chunks |
| History | Discovered by Gregor in 1791; named by Klaproth in 1795. Impure titanium was prepared by Nilson and Pettersson in 1887; however, the pure metal (99.9%) was not made until 1910 by Hunter by heating TiCl4 with sodium in a steel bomb. Titanium is present in meteorites and in the sun. Rocks obtained during the Apollo 17 lunar mission showed presence of 12.1% TiO2. Analyses of rocks obtained during earlier Apollo missions show lower percentages. Titanium oxide bands are prominent in the spectra of M-type stars. The element is the ninth most abundant in the crust of the Earth. Titanium is almost always present in igneous rocks and in the sediments derived from them. It occurs in the minerals rutile, ilmenite, and sphene, and is present in titanates and in many iron ores. Deposits of ilmenite and rutile are found in Florida, California, Tennessee, and New York. Australia, Norway, Malaysia, India, and China are also large suppliers of titanium minerals. Titanium is present in the ash of coal, in plants, and in the human body. The metal was a laboratory curiosity until Kroll, in 1946, showed that titanium could be produced commercially by reducing titanium tetrachloride with magnesium. This method is largely used for producing the metal today. The metal can be purified by decomposing the iodide. Titanium, when pure, is a lustrous, white metal. It has a low density, good strength, is easily fabricated, and has excellent corrosion resistance. It is ductile only when it is free of oxygen. The metal burns in air and is the only element that burns in nitrogen. Titanium is resistant to dilute sulfuric and hydrochloric acid, most organic acids, moist chlorine gas, and chloride solutions. Natural titanium consists of five isotopes with atomic masses from 46 to 50. All are stable. Eighteen other unstable isotopes are known. The metal is dimorphic. The hexagonal α form changes to the cubic β form very slowly at about 880°C. The metal combines with oxygen at red heat, and with chlorine at 550°C. Titanium is important as an alloying agent with aluminum, molybdenum, manganese, iron, and other metals. Alloys of titanium are principally used for aircraft and missiles where lightweight strength and ability to withstand extremes of temperature are important. Titanium is as strong as steel, but 45% lighter. It is 60% heavier than aluminum, but twice as strong. Titanium has potential use in desalination plants for converting sea water into fresh water. The metal has excellent resistance to sea water and is used for propeller shafts, rigging, and other parts of ships exposed to salt water. A titanium anode coated with platinum has been used to provide cathodic protection from corrosion by salt water. Titanium metal is considered to be physiologically inert; however, titanium powder may be a carcinogenic hazard. When pure, titanium dioxide is relatively clear and has an extremely high index of refraction with an optical dispersion higher than diamond. It is produced artificially for use as a gemstone, but it is relatively soft. Star sapphires and rubies exhibit their asterism as a result of the presence of TiO2. Titanium dioxide is extensively used for both house paint and artist’s paint, as it is permanent and has good covering power. Titanium oxide pigment accounts for the largest use of the element. Titanium paint is an excellent reflector of infrared, and is extensively used in solar observatories where heat causes poor seeing conditions. Titanium tetrachloride is used to iridize glass. This compound fumes strongly in air and has been used to produce smoke screens. The price of titanium metal (99.9%) is about $1100/kg. |
| Uses | Ammonium hexafluorotitanate is used as an anti-corrosive cleaning agent. It is also used for the production of ceramics and glass. Further, it is used in the preparation of synthetic gems. |
| Safety Profile | Poison by intravenous route. See also FLUORIDES, AMMONIA, and TITANIUM COMPOUNDS. When heated to decomposition it emits very toxic fumes of Fand NOx,. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1.00/1kg |
VIP2Y
|
Hebei Duling International Trade Co. LTD
|
2023-01-13 | |
| $10.00/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-11-01 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $100.00/1g |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-06-12 | |
| $1.00/1kg |
VIP6Y
|
Career Henan Chemical Co
|
2018-12-20 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 | ||
| $1.00/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-05-20 | |
| $1.00/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-05-19 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China