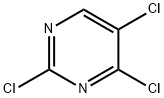
2,4,5-Trichloropyrimidine-Application
Dec 30,2019
The reaction of 2,4,6-trichloropyrimidine 1 with a variety of 4-substituted anilines 2 has been investigated. Monosubstitution occurs readily for all anilines except those containing strongly electron-withdrawing groups. The yields of the isomeric products 3 and 4 parallel the Hammet constants of the ring substituents. The main product when ethanol was used as the solvent was the 4-substituted-2,6-dichloropyrimidine 3. Spectral and X-ray data confirmed this assignment. However, a solvent dependence on the 3:4 ratio was demonstrated. In some cases, excess aniline under forcing conditions led to 2,4-disubstituted products[1].
2,4,5-Trichloropyrimidine is used in the synthesis of potent and selective anaplastic lymphoma kinase (ALK-5) inhibitors, used as an anti-tumor treatment. It is also used in the synthesis of EGFR inhibitors[2,3].
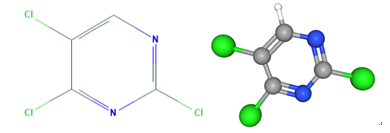
Fig 1. Chemical structure formula and three-dimensional structure of 2,4,5-Trichloropyrimidine
2,4,5-Trichloropyrimidine is applied In selective Suzuki coupling with arylboronic acids has been reported to give 6-arylpyrimidines.
A method for producing a 2,4,5-Trichloropyrimidine compound represented by the formula A (2), characterized in that, in the presence of an organic base of formula (1) dihydroxypyrimidine with a sulfonyl chloride compound represented by hydrogen and is selected from , thionyl chloride, phosgene, phosphorus oxychloride, phosphorus pentachloride and phosphorus trichloride at least one chlorinating agent, in the formula, R represents a hydrogen atom; a halogen atom; a mercapto group; a cyano group; a nitro group ; may be selected from halogen atoms, a cycloalkyl group having 3-6 carbon atoms, 6-14 carbon atoms, an aryl group, an aryl of 3-8 carbon atoms, heteroaryl group, alkoxy group having 1-3 carbon atoms , thioalkyl, 6-14 carbon atoms, an aryl thio group, a cyano group, a nitro group, a C 1-8 1-3 2-14 carbon atoms di-substituted amino group and two carbon atoms of 2-14 a substituted carbamoyl group substituted with at least one substituent of alkyl; alkoxy; alkenyl; alkynyl; carbon atoms may be selected from an alkyl group, an alkenyl group having a carbon number of 1 to 6 2-4 a cycloalkyl group having 5-6 carbon atoms, a halogen atom, an alkoxy group having 1-3 carbon atoms, 2-4 carbon atoms, alkynyl group having 2-14 carbon atoms, two substituted amino, nitro, cyano group of 2-14 carbon atoms and Disubstituted carbamoyl group substituted with at least one substituent aryl group; or may be selected from alkyl, benzyl, 6-10 carbon atoms, an aryl group, a halogen atom, a carbon atom number of 1 to 4 carbon atoms, number 1-3 alkoxy group, a nitro group, a cyano group and 2-14 carbon atoms in the disubstituted amino group substituted with at least one substituent group is heteroaryl, the formulas, R means as defined above.
2,5-Dichloro-N-[2-(isopropylsulfonyl)phenyl]pyrimidin-4-amine was prepared from 2-(isopropylsulfonyl)aniline and 2,4,5-trichloropyrimidine via a coupling reaction in the catalysis of palladium acetate. Meanwhile, 1-chloro-5-isopropoxy-2-methyl-4-nitrobenzene reacted with pyridin-4-ylboronic acid via a Suzuki coupling in dioxane and water to give 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine with a yield of 92.4%. Then the latter was reduced by hydrogenation with PtO2(10%, w/w) as the catalyst to afford 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline dihydrochloride. Then 4 was subjected to the nucleophilic reaction with compound 8 to give ceritinib with a total yield of 57.2%(based on 6), and a purity of 99.94%. By the way, if compound 4 reacted with the free base of 8, the regio-isomer of 1, 2-[4-(4-amino-5-isopropoxy-2-methylphenyl)piperidin-1-yl]-5-chloro-N-[2-(isopropylsulfonyl)phenyl]pyrimidin-4-amine was obtained, which could be separated completely with 1 in HPLC.
References
[1] Delia T J , Meltsner B R , Schomaker J M . 2,4,6‐Trichloropyrimidine. Reaction with sodium amide[J]. Journal of Heterocyclic Chemistry, 1999, 36(5):1259-1261.
[2] Richard Ducray.; Pascal Boutron.; Myriam Didelot.; Hervé Germain.; Franck Lach.; Maryannick Lamorlette.; Antoine Legriffon.; Mickael Maudet.; Morgan Ménard.; Georges Pasquet.; Fabrice Renaud.; Iain Simpson.; Gail L. Young. A versatile route to 3-(pyrimidin-4-yl)-imidazo[1,2-a]pyridines and 3-(pyrimidin-4-yl)-pyrazolo[1,5-a]pyridines. Tetrahedron Letters. 2010, 51 (36), 4755-4758.
[3] Eugen F. Mesaros.; Jason P. Burke.; Jonathan D. Parrish.; Benjamin J. Dugan.; Andrew V. Anzalone.; Thelma S. Angeles.; Mark S. Albom.; Lisa D. Aimone.; Matthew R. Quail.; Weihua Wan.; Lihui Lu.; Zeqi Huang.; Mark A. Ator.; Bruce A. Ruggeri.; Mangeng Cheng.; Gregory R. Ott.; Bruce D. Dorsey. Novel 2,3,4,5-tetrahydro-benzo[d]azepine derivatives of 2,4-diaminopyrimidine, selective and orally bioavailable ALK inhibitors with antitumor efficacy in ALCL mouse models. Bioorganic & Medicinal Chemistry Letters. 2011, 21 (1), 463-466
- Related articles
- Related Qustion
Vitamin E succinate is one of many substances known as an antioxidant. These substances help in the protection of your body’s cells. When you have an antioxidant in your system, unstable molecules called free radicals cannot penetrate your....
Dec 30,2019APIEthyl lactate (EL), also known as lactic acid ethyl ester, is a monobasic ester formed from lactic acid and ethanol, commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl....
Dec 30,2019Flavors and fragrances2,4,5-Trichloropyrimidine
5750-76-5You may like
2,4,5-Trichloropyrimidine manufacturers
- 2,4,5-Trichloropyrimidine
-

- $30.00 / 1kg
- 2024-05-30
- CAS:5750-76-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
- 2, 4, 5-Trichloropyrimidine
-

- $10.00 / 1kg
- 2024-04-24
- CAS:5750-76-5
- Min. Order: 1kg
- Purity: 99.6%
- Supply Ability: 100000
- 2,4,5-Trichloropyrimidine
-

- $5.00 / 1KG
- 2024-04-08
- CAS:5750-76-5
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available