2-Chlorobenzaldehyde as a Metabolite of Riot Control Agent CS: Implications for Public Health
Dec 28,2023
General Description
2-Chlorobenzaldehyde is a chemical compound with moderate lipophilicity and physical properties such as a boiling point of 212 °C, melting point of 11.9 °C, and density of 1.2483 g/cm³. It is a metabolite of the riot control agent CS and has been shown to be absorbed through the skin and eliminated primarily through the urine. However, it is associated with toxic effects in animals and potential risks to humans, including neurotoxicity, dermatotoxicity, and potential toxic pneumonitis when inhaled. Ingestion can also cause abdominal pain and burning sensations. Animal studies show acute toxicity through different routes of exposure. These findings highlight the importance of handling 2-chlorobenzaldehyde with caution and implementing proper safety measures in its use and management.

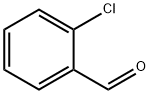
Figure 1. 2-Chlorobenzaldehyde
Physical and Chemical Properties
2-Chlorobenzaldehyde is a chemical compound with a molecular weight of 140.56 g/mol. It has a computed XLogP3 value of 2.3, indicating moderate lipophilicity. The compound does not have any hydrogen bond donor groups but has one hydrogen bond acceptor group. It contains one rotatable bond and a heavy atom count of 9. The exact and monoisotopic masses are both 140.0028925 g/mol. Physically, 2-chlorobenzaldehyde is a clear colorless to yellowish liquid with a penetrating odor. It has a boiling point of 212 °C and a melting point of 11.9 °C. The flash point is 87 °C, and its vapor pressure at 25 °C is 0.04 kPa. The compound is slightly soluble in water but soluble in ethanol, diethyl ether, acetone, benzene, and carbon tetrachloride. It has a density of 1.2483 g/cm³ at 20 °C and a refractive index of 1.5662 at 20 °C. 2-Chlorobenzaldehyde is stable under recommended storage conditions and does not have a specific shelf life mentioned. It decomposes when heated, emitting toxic fumes of chloride. The compound belongs to the chemical class of benzaldehydes. 1
Pharmacokinetics
2-Chlorobenzaldehyde is a metabolite of the riot control agent CS, generated through CS hydrolysis in both in vitro and in vivo conditions. However, limited quantitative data exists regarding its percutaneous absorption, cutaneous biotransformation, and elimination. A study in rats investigated the pharmacokinetics of (14)C-labeled 2-chlorobenzaldehyde following intravenous, intraperitoneal, and cutaneous administrations. Results showed rapid plasma radioactivity decline after systemic administration, while cutaneous application led to slow skin penetration and a subsequent gradual decline in plasma radioactivity over three days. Metabolite analysis revealed that most of the radioactivity was excreted in urine, with 2-chlorohippuric acid identified as the principal metabolite. Notably, the qualitative and quantitative metabolic patterns of urinary excreted metabolites were similar between cutaneous and systemic administrations, suggesting no significant storage in the skin or skin toxicity following cutaneous application. Additional research in rabbits and rats further supported the metabolic pathways and urinary excretion of mercapturic acids for substituted benzaldehydes. These findings contribute to our understanding of the pharmacokinetic profile of 2-chlorobenzaldehyde as a potential consequence of CS exposure. 2
Toxicity
2-Chlorobenzaldehyde, a chemical used as an intermediate in the production of dyes and organic compounds, is associated with toxic effects in animals and potential risks to humans. When moist skin is exposed to the riot control agent CS, 2-chlorobenzaldehyde may be generated through hydrolysis. It has been identified as a neurotoxin affecting the central nervous system, a dermatotoxin causing skin burns, and a potential cause of toxic pneumonitis when inhaled. Inhalation can lead to symptoms such as coughing, sore throat, and burning sensations in the respiratory tract, while skin contact may result in redness and pain. Ingestion can cause a burning sensation in the mouth and stomach, along with abdominal pain. Animal studies have demonstrated acute toxicity, with symptoms including somnolence, tremors, and gastrointestinal hypermotility. The LD50 values in animals indicate varying degrees of acute toxicity through different routes of exposure. Additionally, o-chlorobenzaldehyde has shown genotoxicity in yeast tests. These findings underscore the importance of handling 2-chlorobenzaldehyde with caution and implementing proper safety measures in its use and management. 3
Reference
1. 2-Chlorobenzaldehyde. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 6996.
2. Rietveld EC, Plate R, Seutter-Berlage F. Mechanism of formation of mercapturic acids from aromatic aldehydes in vivo. Arch Toxicol. 1983 Mar;52(3):199-207.
3. PubChem Annotation Record for o-Chlorobenzaldehyde. National Center for Biotechnology Information, 2023, Source: Hazardous Substances Data Bank.
- Related articles
- Related Qustion
- 2-Chlorobenzaldehyde: Chemical Properties, Pharmacokinetic Characteristics and Toxicity Apr 25, 2024
2-Chlorobenzaldehyde, rapidly excreted in rat urine, undergoes metabolism to 2-chlorohippuric acid and benzyl alcohols, with potential toxicity concerns in CS-related incidents.
- 2-Chlorobenzaldehyde: The synthesis and application Apr 12, 2023
2-Chlorobenzaldehyde (CAS No.: 89-98-5), also known as Amlodipine Impurity T, with molecular formula of C7H5ClO and molecular weight of 140.57, is used as medicine and dye intermediate.
p-Coumaric acid is a compound found in plants and mushrooms and exhibits significant antioxidant and anticancer activities.....
Dec 28,2023APIOzanimod Hydrochloride is prepared from the eastern fragment joined to the western fragment to form a central oxadiazole ring and through a chemical reaction.....
Dec 28,2023Inhibitors2-Chlorobenzaldehyde
89-98-5You may like
2-Chlorobenzaldehyde manufacturers
- 2-Chlorobenzaldehyde
-

- $1.00 / 1g
- 2024-06-10
- CAS:89-98-5
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- 2-Chlorobenzaldehyde
-

- $0.00 / 200KG
- 2024-06-07
- CAS:89-98-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500mt/year
- 2-Chlorobenzaldehyde
-

- $79.00 / 1kilograms
- 2024-06-07
- CAS:89-98-5
- Min. Order: 1kilograms
- Purity: 99%
- Supply Ability: 100tons