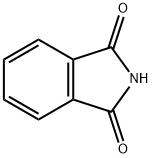
Preparation method and application of phthalimide
Apr 22,2022
Background and overview
Phthalimide, pure product is white and crisp crystal, industrial product is light yellow amorphous block, soluble in alkali and glacial acetic acid, insoluble in water, slightly soluble in heated chloroform, benzene and ether , in alcohol. This product is an intermediate of many fine chemicals such as dyes, pesticides, medicines, rubber auxiliaries, etc. Wait.
Preparation
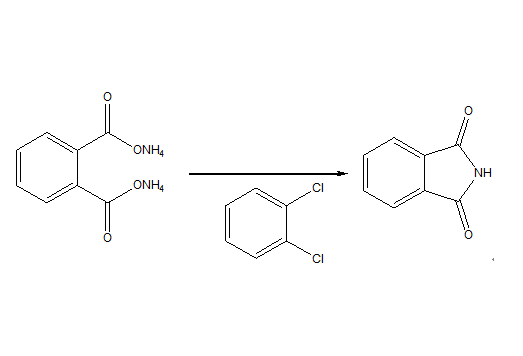
Diammonium phthalate to phthalimide450g (345 ml) o-dichlorobenzene is placed into a 1 -liter four-necked round-bottom flask and heated up to 150 to 155°C. At this temperature, 545 g diammonium phthalate water solution (47%) pre-heated at 70°C is then added into this flask continuously within 4 hours. Water is directly distilled and separated from the system. During the process, part of diammonium phthalate is transferred to phthalmide, producing some ammonium and additional water which is also distilled. After the addition of diammonium phthalate solution is completed and no more water is distilled, the temperature inside the flask is increased slowly within 3 hours to make o-dichlorobenzene reflux. Water resulted from this process is further distilled away. The reaction is ended when the solution becomes clear. The reaction solution is then cooled down to the room temperature upon stirring. Phthalimide is crystallized, filtered, separated and then dried inside a vacuum drying oven.The reaction gives 160 g phthalimide with a purity higher than 99%, in a yield of 85%.
Use
Can be used to prepare 2-benzyl-isoindole-1,3-dione
A three-necked flask was equipped with a thermometer, a reflux condenser and a stirring device, and amine (2.0 mmol), formamide (2.4 mmol) and sulfated polyborate (10 wt.%) were added in sequence, and the stirring was turned on and the reaction solution was heated to about 120 °C. , after 4 hours of incubation reaction, the reaction is monitored by thin-layer chromatography until the reaction is complete, and if any raw materials remain, the incubation reaction is continued. After the reaction was complete, the mixture was cooled to room temperature and quenched with water; the precipitated solid was filtered with a vacuum pump, washed with water (3 x 5 mL), dried under vacuum and recrystallized from ethanol to give pure organic product. For liquid product, the reaction mixture was diluted with water and extracted with ethyl acetate (3×5 mL). The combined organic layers were washed with water, dried over sodium sulfate, filtered and evaporated under reduced pressure to give the crude product, which was purified by column chromatography using silica as stationary phase and ethyl acetate/petroleum ether as mobile phase. To recycle the catalyst, the aqueous quench and wash liquors were stored and evaporated, then dried under vacuum. The solids thus obtained are used as in subsequent runs without significant loss of yield.
Can be used to prepare 2-hydroxymethyl-isoindole-1,3-dione
In a 2000ml four-necked flask equipped with mechanical stirring and a thermometer, add 147g (1mol) phthalimide, 32g paraformaldehyde and 1470g purified water, then the flask is equipped with a reflux condenser and a heating mantle, turn on the stirring, and start heating. When the temperature rises to 98°C, droplets at the lower end of the reflux condenser begin to reflux. Continue to heat, and when the temperature reaches 100 °C, the lower end of the reflux condenser is trickling back. The heating was controlled to keep the reaction at reflux and the reaction was continued for 3 hours. After that, the heating device was removed, and it was naturally cooled to room temperature. Then suction filtration, and the filter cake was dried to obtain 175 g of white needle-like crystalline powder. The liquid chromatography content was 99.50%, and the molar yield was 98.87% in terms of phthalimide.
Can be used to prepare intermediates of various fine chemicals such as organic synthetic dyes, pesticides, medicines, rubber auxiliaries, etc.
References
[1] WO2015/91965,2015, A2
[2] Organic Preparations and Procedures International,2021, vol. 53, # 4, p. 369 – 378
[3] CN113292478,2021, A
- Related articles
- Related Qustion
Phthalimide
85-41-6You may like
- Phthalimide
-

- $100.00 / 1KG
- 2024-05-10
- CAS:85-41-6
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 20 tons
- Phthalimide
-

- $25.00 / 1kg
- 2023-11-15
- CAS:85-41-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons
- Phthalimide
-

- $0.00 / 1KG
- 2023-09-06
- CAS:85-41-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg