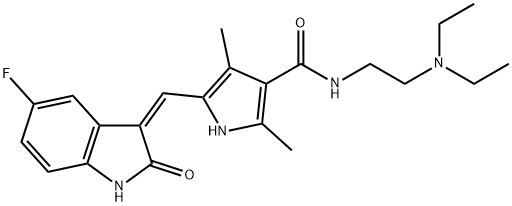
An oral multiple tyrosine kinase receptor inhibitor: Sunitinib
Oct 31,2023
Description
Sunitinib is a yellow solid with the chemical formula C22H27FN4O2. This compound is Slightly soluble in Chloroform and Methanol. Sunitinib is a member of pyrroles and a monocarboxylic acid amide. Based on its good anti-tumor properties, it is widely used in the treatment of various cancers.
Use
Sunitinib was first approved by the U.S. FDA in January 2006 for advanced renal cell carcinoma, also as a second-line therapy for patients whose gastrointestinal stromal tumor is resistant to imatinib. This drug has a role as an angiogenesis inhibitor, an antineoplastic agent, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, a vascular endothelial growth factor receptor antagonist, an immunomodulator, and a neuroprotective agent. Currently, Sunitinib is used to treat gastrointestinal stromal tumors (GIST; a type of tumor that grows in the stomach, intestine (bowel), or esophagus (tube that connects the throat with the stomach) in people with tumors that were not treated successfully with imatinib (Gleevec) or people who cannot take imatinib[1]. Sunitinib is also used to treat advanced renal cell carcinoma (RCC, a type of cancer that begins in the cells of the kidneys).
Mechanism of action
Sunitinib malate is an oral receptor TKI of VEGF receptor 1 (VEGFR‐1), VEGFR‐2, VEGFR‐3, PDGF receptor‐α(PDGFR‐α), PDGFR‐β, and other receptor tyrosine kinases. Sunitinib inhibits cellular signaling by targeting multiple receptor tyrosine kinases (RTKs). These include all receptors for platelet-derived growth factors (PDGF-Rs) and vascular endothelial growth factor receptors (VEGFRs), which play a role in both tumor angiogenesis and tumor cell proliferation. The simultaneous inhibition of these targets therefore reduces tumor vascularization and triggers cancer cell apoptosis and thus results in tumor shrinkage.

Side effects
TKI-induced hypothyroidism (i.e., deficiency of thyroid hormone) is characterized by several symptoms, such as tiredness, poor ability to tolerate colds, and weight gain[2]. Sunitinib, for instance, is suspected of causing thyroid dysfunction more often than other TKIs. The most common adverse events associated with sunitinib therapy are fatigue, diarrhea, nausea, anorexia, hypertension, yellow skin discoloration, hand-foot skin reaction, and stomatitis. It is usually well tolerated and nephrotoxicity is very rare.
References
[1] Motzer R, et al. Sunitinib: Ten Years of Successful Clinical Use and Study in Advanced Renal Cell Carcinoma. The Oncologist, 2016; 22: 41–52.
[2] Shu M, et al. Hypothyroidism Side Effect in Patients Treated with Sunitinib or Sorafenib: Clinical and Structural Analyses. PLOS ONE, 2016.
- Related articles
- Related Qustion
- Sunitinib in the Treatment of Thyroid Cancer Feb 2, 2024
Sunitinib is a multi-targeted tyrosine kinase inhibitor used for various cancers, including thyroid cancer with specific gene rearrangements, demonstrating anti-tumor activity in clinical trials.
Trospium chloride, a quaternary amine with anticholinergic properties, is used for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.....
Oct 31,2023APICamphor is highly lipid soluble and easily crosses the blood–brain barrier. The natural form of camphor is D-camphor, while the synthetic version is a mixture of the D and L enantiomers.....
Nov 1,2023Organic reagentsSunitinib
557795-19-4You may like
- Acid phosphatases and Phosphatase, Acid from wheat germ
May 27, 2024
- The structure and Biological function of Cytochrome C
May 17, 2024
- Is CJC-1295 DAC same as CJC-1295?
May 16, 2024
- Sunitinib
-

- $50.00 / 10G/Bag
- 2024-05-31
- CAS:557795-19-4
- Min. Order: 1G/Bag
- Purity: 0.99
- Supply Ability: 100kg
- Sunitinib
-
- $1.00 / 1kg
- 2024-05-31
- CAS:557795-19-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 200
- Sunitinib
-

- $0.00 / 1kg
- 2024-05-09
- CAS:557795-19-4
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton