Linalool: Properties, Synthesis and Uses
Mar 5,2024
Properties of Linalool
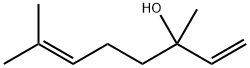
Linalool, also known as 3,7-dimethyl-1,6-octadien-3-ol, β-linalool, linalyl alcohol, 2,6- dimethyl-2,7-octadien-6-ol, 2,6-dimethyl-2,7-octadiene-6-ol, 2,6-dimethylocta-2,7-dien- 6-ol, 3,7-dimethylocta-1,6-dien-3-ol, dl-3,7-dimethyl-3-hydroxy-1,6-octadiene, phantol, 3,7-dimethyl-1,6-octandien-3-ol, R/S-linalool, (S)-linalool, (+)-linalool, (–)-β-linalool, (S)-linalool, l-linalool, (±)-linalool and (+)-β-linalool, is a derivative of acetylene and acetone.
Linalool is a colorless liquid with a flash point of 76 °C. It is insoluble in water and
glycerin, and can be miscible with ethanol and ether. Linalool is the main component
of natural eucalyptus oil, galaxie oil, rosewood oil and so on, and all of them are
levorotatory isomers, while those present in eucalyptus oil and sesame oil are dextrorotatory isomers. The physical properties of these two isomers are listed in Table 4.6.

Most of the linalool and its esters have a beautiful and pleasant floral aroma. It has a
chiral carbon atom with different aromas for linalool with different optical rotations.
Its unique aroma quality makes it an important part of many fragrance formulas,
which can be used to blend various flavors such as lily, clove and orange blossom.
In addition, it is also an important raw material for the synthesis of linalool flavor
compounds and vitamins E and A.
Synthesis of Linalool
Earlier, linalool was mainly obtained from many natural essential oils. With the increasing use and dosage of linalool and its derivatives, the production of essential oils cannot keep up with the development needs, so the production by synthetic methods has attracted attention. Synthesis of linalool by selective catalytic hydrogenation of dehydrolinalool is shown as follows:

Generally, elements such as palladium, rhodium, platinum and nickel are selected
as catalysts, and the carrier is usually Al2O3 or CaCO3. It is further modified with lead,
bismuth, manganese and pyridine, quinoline and sulfur compounds. Both the conversion of dehydrolinalool and the yield of linalool were more than 99% at a reaction
temperature of 60 °C and a pressure of 0.4 MPa.
Uses of Linalool
Linalool is a fragrance and flavor substance. It is a blending raw material for various artificial essential oils. Its unique aroma quality makes it an important part of many fragrance formulas, which can be used to blend various flavors such as lily, clove and orange blossom. In addition, it is also an important raw material for the synthesis of geranyl acetone and β-ionone, isophytol, vitamin A and E and β-carotene.
- Related articles
- Related Qustion
- Anticonvulsant properties of linalool Nov 12, 2019
Linalool is a component of many essential oils, including orange, lavender, rose, rosewood, and coriander. The main problem the Cosmetics Database has with linalool is the risk of skin irritation and allergic reactions.
Boric acid is a water-soluble white compound and occurs naturally. Its molecular formula is H3BO3.....
Mar 5,2024Inorganic chemistryPhysiological sodium chloride solution normally has a shelf life of 24 months. Deterioration and bacterial growth will usually occur if it exceeds the validity period.....
Mar 5,2024Biochemical EngineeringLinalool
78-70-6You may like
- Is dimethyl disulfide a natural product?
May 22, 2024
- What is the effect of water on 3-aminopropyltriethoxysilane (APTES)?
May 17, 2024
- The solubility of Succinic anhydride
May 11, 2024