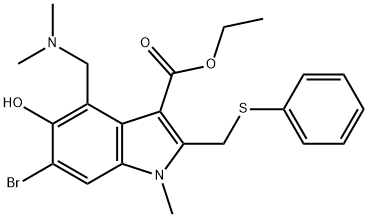
New coronavirus drug-Arbidol
Nov 1,2021
Arbidol is an antiviral drug first launched in Russia in 1993. It is clinically used to prevent and treat influenza and other acute viral respiratory infections. It is suitable for adults and children with influenza A, influenza B, acute viral respiratory infections, and severe Prevention and treatment of acute respiratory disease syndrome and complicated bronchitis and pneumonia. The latest research shows that Arbidol can effectively inhibit the new pneumonia coronavirus.

Uses
Arbidol is suitable for adults and children with influenza A, influenza B, acute viral respiratory infections, severe acute respiratory syndrome, including the prevention and treatment of complicated bronchitis and pneumonia. Early use of Arbidol hydrochloride after the onset of influenza can significantly shorten the duration of the disease and reduce the severity of symptoms. It is useful for improving cough, headache, fever, chills, sweating, sore throat, muscle aches and fatigue, etc. The symptoms have obvious effects, and the safety is good, suitable for clinical promotion and use.
Toxity
This product is a medicine for the prevention and treatment of influenza. It blocks the replication of the virus by inhibiting the fusion of the lipid membrane of the influenza virus with the host cell. Studies have shown that in vitro cell culture of this product can directly inhibit the replication of influenza A and B viruses, and in vivo animal experiments can reduce the mortality of influenza virus-infected mice. This product still has interferon inducing effect.
Pharmacokinetics
A single dose of arbidol hydrochloride 200 mg orally in healthy subjects, the concentration of arbidol in plasma reached the peak value (417.8±240.7ng/ml) at about 1.63 hours, the half-life of arbidol was 10.55±4.01h, and the AUC0-t was 2725.8±1181.0ngh/ml, AUC0-∞ is 2857.4±1311.3ngh/ml. In addition, according to animal pharmacokinetics, rats were quickly absorbed after intragastric administration of the drug, plasma tmax was 20min; 150mg/kgCmax was 5.9μg/ml, t1/2 was 6.7h; 300mg/kgCmax was 12.9μg/ml, t1 /2 is 15.0h, and the absolute bioavailability is 35.6%. The drug is distributed throughout the body, with the highest concentration in the liver, followed by the thymus, kidney and brain. 48 hours after administration, 40% of the drug was excreted in its original form, of which 38.9% was excreted in feces and less than 0.12% excreted in urine.
- Related articles
- Related Qustion
- Arbidol: History, Pharmacokinetics and Toxicity Apr 8, 2024
Arbidol, a Russian-developed antiviral, shows promise in treating respiratory diseases, but requires further research for long-term safety and efficacy.
- Arbidol: mechanism of action, clinical applications and safety Nov 14, 2023
Arbidol is a broad-spectrum antiviral drug that inhibits virus replication and entry, showing efficacy against respiratory viral infections with a good safety profile.
Fludioxonil is a new type of pyrrole non-systemic, contact-killing broad-spectrum fungicide. As a seed treatment fungicide, the suspension seed coating agent can control many diseases.....
Oct 29,2021Pesticide IntermediatesAllyl formate is a clear, colorless liquid. It is slightly soluble in water. It is reported to cause liver and kidney injury in animals. The most common effect in humans following occupational exposure to allyl formate exposure is upper res....
Nov 1,2021Organic ChemistryArbidol
131707-25-0You may like
- Arbidol
-

- $0.00 / 1kg
- 2024-05-09
- CAS:131707-25-0
- Min. Order: 1kg
- Purity: 99%HPLC
- Supply Ability: 20 tons
- Umifenovir
-

- $80.00 / 1KG
- 2023-08-16
- CAS:131707-25-0
- Min. Order: 1KG
- Purity: >99%
- Supply Ability: 20tons
- Arbidol
-

- $48.00 / 1KG
- 2023-05-04
- CAS:131707-25-0
- Min. Order: 1公斤
- Purity: 99%
- Supply Ability: 200tons