Reactions of Cobalt carbonyl
Feb 24,2022
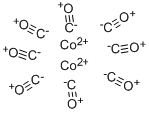
Dicobalt octacarbonyl is a metal compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry.It is the precursor to a hydroformylation catalyst, cobalt tetracarbonyl hydride.Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, though multiple distinct structural arrangements are known.Some of the carbonyl ligands are highly labile. The compound is highly reactive towards alkynes, and is sometimes used as an alkyne protecting group. As the cobalt-alkyne complex, it plays a role in promoting both the Nicholas reaction and the Pauson–Khand reaction.

Reactions
Reduction
Dicobalt octacarbonyl is reductively cleaved by alkali metals and relatived reagents. The resulting alkali metal salts protonate to give tetracarbonyl cobalt hydride:
Co2(CO)8 + 2 Na → 2 NaCo(CO)4
NaCo(CO)4 + H+ → HCo(CO)4 + Na+
Reactions with electrophiles
Halogens and related reagents cleave the Co-Co bond to give pentacoordinated halotetracarbonyls:
Co2(CO)8 + Br2 → 2 BrCo(CO)4
Cobalt tricarbonyl nitrosyl is produced by treatment of dicobalt octacarbonyl with nitric oxide:
Co2(CO)8 + 2 NO → 2 Co(CO)3NO + 2 CO
Nicholas reaction
The Nicholas reaction is a substitution reaction whereby an alkoxy group located on the α-carbon of an alkyne is replaced by another nucleophile.The alkyne reacts first with dicobalt octacarbonyl, from which is generated a stabilized propargylic cation that reacts with the incoming nucleophile and the product then forms by oxidative demetallation.
Pauson–Khand reaction
The Pauson–Khand reaction,in which an alkyne, an alkene, and carbon monoxide cyclize to give a cyclopentenone, can be catalyzed by Co2(CO)8,though newer methods that are more efficient have since been developed:

- Related articles
- Related Qustion
Acetone oxime (acetoxime) is the organic compound with the formula (CH3)2CNOH. It is the simplest example of a ketoxime. It is a white crystalline solid that is soluble in water, ethanol, ether, chloroform, and ligroin. It is used as a reag....
Feb 23,2022Organic ChemistryMannitol is an alcohol produced by the reduction of mannose. It is absorbed unreliably from the GI tract and therefore has to be given by i.v. injection; bolus doses of 0.25–1 g kg–1 are used. Initially it stays within the intravascular spa....
Feb 24,2022Biochemical EngineeringCobalt carbonyl
10210-68-1You may like
- Tris(dibenzylideneacetone)dipalladium: uses and Health effect
May 9, 2024
- polypropylene vs polycarbonate
Mar 18, 2024
- What is sodium chlorite used for?
Mar 16, 2024
- Cobalt carbonyl (Dicobalt octacarbonyl)
-

- $890.00 / 100g
- 2024-04-20
- CAS:10210-68-1
- Min. Order: 1g
- Purity: 90
- Supply Ability: 500 Kg
- Cobalt carbonyl
-

- $35.00 / 1kg
- 2024-03-29
- CAS:10210-68-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Cobalt carbonyl
-

- $100.00 / 1kgkg
- 2023-10-09
- CAS:10210-68-1
- Min. Order: 1kgkg
- Purity: 99%
- Supply Ability: 100 tons