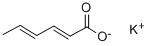
Potassium sorbate: Chemical synthesis, application, and toxicity
Mar 15,2023
Staphylococcus and Salmonella.Its appearance is as follows:

Figure 1 Appearance of potassium sorbate.
The melting point of potassium sorbate is 270 ° C and the density is 1361 g/cm3. The solubility in water is 1 M at 20 °C, and its aqueous solution is clear, colorless to fatly yellow solution, which is soluble in propylene glycol (5.8 g/100 mL) and ethanol (0.3 g/10 mL). Potassium sorbate is white to light yellow flake crystal, crystalline powder or granular. It is easy to absorb moisture and oxidize and decompose when stored in the air for a long time. Potassium sorbate is soluble in water, propylene glycol and ethanol. Sorbic acid (potassium) is stable in a sealed state, easy to absorb water when exposed to moist air, oxidize and stain. Potassium sorbate has good thermal stability, and its decomposition temperature is up to 270 ℃.
The potassium sorbate has antiseptic and antibacterial activities, and are basically non-toxic to human body when used at common concentrations. Therefore, these compounds can be used as food additives. Potassium sorbate is a preservative for food. It can effectively inhibit the activity of molds, yeasts and aerobic bacteria, and also prevent the growth and reproduction of harmful microorganisms such as Botox, Staphylococcus, Salmonella, but it has little effect on beneficial microorganisms such as anaerobic bacillus and lactobacillus acidophilus. Its inhibiting effect on development is stronger than that on sterilization, so as to effectively prolong the storage time of food and maintain the flavor of original food. Its antiseptic effect is 5~10 times that of the same product sodium benzoate. It is widely used in food, medicine, rubber, paper, animal feed, cosmetics, paint, tobacco, beverage and other industries [2].
The most commonly used analytical methods for the quality assurance of sorbates in food are spectrophotometry, gas chromatography–mass spectrometry (GC-MS), liquid chromatography–mass spectrometry (LC- MS), capillary electrophoresis, and high-performance liquid chroma- tography (HPLC) equipped with an ultraviolet detector or a UV-diode array detector (DAD). Among them, GC-MS can be reliable method for measurement of PS but Pylypiw et al. and Esfandiari et al. introduced HPLC as a more accurate and fastest method for determination of sor- bate in foodstuff [3].
Metabolism and pharmacokinetics
The results of metabolic studies indicated that potassium sorbate is completely absorbed after oral administration and subsequently distributed in the body. It can be metabolized and oxidized into carbon dioxide (CO2) and H2O like hexanoic acid in human body. 80–86% of it exhaled through lungs as CO2 and 2–10% of PS excreted via urine as urea and in lower concentrations as muconic and sorbic acid. Excretion via the lung is completed after 10 h of application [4]. Potassium sorbate can form covalent binding with Lys-190 of human serum albumin (HSA) residues and can result in conformational changes of the protein, oxidative stress, amyloid fibril formation of albumin and in- terferences with human diseases such as diabetic and obesity [5]. Potassium sorbate is absorbed via a diffusion process in the stomach and it can be dissociated into its constituents (potassium and sorbate) and absorbed through small intestine in the form of sorbic acid [6]. Re- garding the tissue distribution, 85% of the sorbic acid was metabolized to carbon dioxide, 3% of it was remained in internal organs, 3% of it was found in the skeletal muscles, approximately 2% was excreted in the urine as urea and 0.4% in the feces, and 6.6% was found in the other parts of the body.
Toxicity and side effects
It should be noted that even though
[2]Taghavi et al. Potassium sorbate as an AGE activator for human serum albumin in the presence and absence of glucose. International Journal of Biological Macromolecules, 2013, 62: 146–154.
[3]Esfandiari et al. Simultaneous determination of sodium benzoate, potassium sorbate and natamycin content in Iranian yoghurt drink (Doogh) and the associated risk of their intake through Doogh consumption. Iranian Journal of Public Health, 2013, 42: 915.
[4]Dehghan et al. Pharmacokinetic and toxicological aspects of potassium sorbate food additive and its constituents. Trends in Food Science & Technology, 2018, 80: 123-130.
[5]Deshpande. Handbook of food toxicology. 2003, CRC Press.
[6]Walker. Toxicology of sorbic acid and sorbates. Food Additives & Contaminants, 1990, 7, 671–676.
[7]Lebe et al. Effects of preservatives in nasal formulations on the mucosal integrity: An electron microscopic study. Pharmacology, 2004, 72, 113–120.
[8]Fisher et al. Cutaneous reactions to sorbic acid and potassium sorbate. Cutis, 1980, 25 350, 352, 423.
- Related articles
- Related Qustion
- Potassium Sorbate: A Comprehensive Insight into its Synthesis, Composition, Applications, Toxicity, and Safety Precautions May 9, 2024
Potassium sorbate has garnered significant attention for its versatile applications and safety considerations.
- Potassium Sorbate: Versatile and Effective Food Preservative with Alkylating Potential Jan 5, 2024
Potassium sorbate is a commonly used food preservative with low toxicity and high solubility and exhibits alkylating potential, but further research is needed on its implications for human health.
N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine is a highly mobile synthetic cytokinin.....
Mar 15,2023APIThe 4-[(2,4-Dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid is a chemical linker, which is often used to synthesize peptides. It can improve the diabetic complications.....
Mar 15,2023APIPotassium sorbate
24634-61-5You may like
Potassium sorbate manufacturers
- Potassium sorbate
-

- $20.00 / 1kg
- 2024-05-30
- CAS:24634-61-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000 tons/month
- Potassium sorbate
-

- $18.00 / 10KG
- 2024-05-08
- CAS:24634-61-5
- Min. Order: 1KG
- Purity: 99.9
- Supply Ability: 5000
- Potassium Sorbate
-

- $120.00 / 1kg
- 2024-05-08
- CAS:24634-61-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton