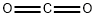The Lewis structure of Carbon dioxide
Nov 8,2023
What is Lewis structure?
The Lewis structure is an image of atoms and atomic bond structures in a molecule that indicate the presence of lone pairs of electrons, named after the American physical chemist Gilbert Newton Lewis. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. Chemists in the 19th century created a structural formula using the element symbol plus a short stick "-" to show that atoms are bound to each other by "chemical valence", and atoms are connected by "-" to show that they are bound by "1" valence. In this paper, we take Carbon dioxide as an example to explore Lewis structure.
The Lewis structure of Carbon dioxide
Carbon dioxide (CO2) is a colorless, odorless gas present throughout the atmosphere and is an essential compound for life on Earth. The Lewis structure of CO2 is shown below:

The carbon-oxygen ratio in a CO2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom. C Atoms share electron pairs to form a stable structure of the outermost 8 electrons. "=" means that the C and O atoms share two pairs of electrons.There are two double bonds around the carbon atom. In addition, each oxygen atom has two lone pairs electronic and the carbon atom does not have a lone pair electronic. Also, there are no charges in oxygen atoms and carbon atoms.
The CO2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. In particular, all these two double bonds are located around carbon atoms. Therefore, the hybridization of carbon is sp. The presence of a sigma bond and valence electron pairs repelling each other forces them to move to the opposite side of the carbon atom, resulting in this geometric shape. As a result, the carbon atom takes on a linear molecular shape with symmetric charge distribution. The bond angle of carbon dioxide is 180°.
- Related articles
- Related Qustion
- Dry ice:Chemical formula,Uses,Safety Mar 19, 2024
Dry ice is the solid form of carbon dioxide (chemical formula CO2), comprising two oxygen atoms bonded to a single carbon atom.
- Is carbon dioxide a mixture or a pure substance? Feb 29, 2024
Carbon dioxide (CO2) is indeed considered a pure substance. A molecule of carbon dioxide (CO2) is made up of one carbon atom and two oxygen atoms.
- Non-polar molecule with polar bonds: CO2 Dec 19, 2023
Carbon dioxide is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of 180 degrees from either direction.
Both Azithromycin and Amoxicillin are considered broad-spectrum antibiotics, which means they treat a wide range of infections, but azithromycin treats a longer list than amoxicillin.....
Nov 8,2023APIGliclazide is a second-generation, sulfonylurea oral hypoglycemic agent. It can improve blood clotting and postpone the complications of diabetes, and is commonly used in the diabetic II treatment.....
Nov 9,2023APICarbon dioxide
124-38-9You may like
- CARBON DIOXIDE USP/EP/BP
-

- $1.10 / 1g
- 2021-07-29
- CAS:124-38-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min