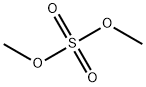
1,2-Dimethoxypropane synthesis
- Product Name:1,2-Dimethoxypropane
- CAS Number:7778-85-0
- Molecular formula:C5H12O2
- Molecular Weight:104.15
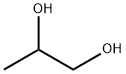
57-55-6
1515 suppliers
$5.00/1g
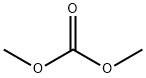
616-38-6
658 suppliers
$5.00/5 g
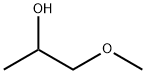
107-98-2
617 suppliers
$16.00/25mL
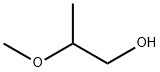
1589-47-5
77 suppliers
inquiry

7778-85-0
139 suppliers
$18.00/25g
Yield:-
Reaction Conditions:
with carbon dioxide in methanol at 200; under 20686.5 - 97743.6 Torr; for 5 h;Sealed tube;
Steps:
4 Example 4.
Experimental condition (MeOH, DMC, C02). A 250 cc Hastelloy pressure vessel was charged with 10 g of propylene glycol (PG, 131 mmol), 50 g of dimethyl carbonate (DMC, 550 mol, 4.2 eq.) and 50 g of MeOH. The vessel was then sealed tightly and affixed to the reactor apparatus, purged x3 with 400 psi of C02, then saturated with CO2 until the pressure remained steady at 400 psi (methanol absorbs considerably amounts of C02). While stirring at 700 rpm, the vessel was heated to 200°C,where the reaction persisted for 5 h; the maximum pressure attained was 1890 psi at this temperature. After that time, the solution was cooled to ambient temperature, gas released, and stirring halted. The resultant brownish solution was then analyzed by GC/MS (70°C initial temp, hold for 4 mm, then 10°C per minute until 300°C, hold for 10 mm). The resultant chromatogram (Figure 1 2A) revealed a small signal at 2.72 mm with m/z of 76.0 (unreacted PG), and two prominent signals at 2.126,2.159 mm bothwith m/z of 90.0, consistent with the monomethylether isomers of PG. Figures 12B and 12C show the mass spectrum of the PG-monoethyl ether isomers A or B.[0058] GC/MS analysis using a HP Innowax column and following inlet and oven temperatureramps: Inlet - 60°C initial, hold for 1 mm, ramp 5°C per mm until 100°C, no hold, ramp 60°C per mm until 250°C; Oven - 70°C initial, hold for 5 mm, ramp 10°C per mm until l5OoC, no hold, ramp 20°Cper minute until 240 mm, no hold. The results are presented in Figures 13A-13D.[0059] FIG. 13A, is a gas chromatogram of the mixed mono- and di-methyl ether products of PG etherification according to an embodiment of the present process. FIG. 13B is the mass spectrum corresponding to the signal at 13.52 minutes in the gas chromatogram detailed in Figure 13A, andspecifying unreacted propylene glycol. FIG 1 3C is the mass spectrum corresponding to the signal at2.502 minutes in the gas chromatogram detailed in Figire 13A, and denoting PG dimethyl ether (1,2-dimethoxypropane). FIG 13D is a mass spectrum corresponding to the signal at 3.158 minutes in the gas chromatogram detailed in Figure 1 3A, and represents isomers of PG monomethyl ether (1-methoxypropan-2-ol and 2-methoxypropan-1 -ol).[0060] Both the chromatograms and corresponding spectra reveal clearly a high rate of conversion ofPG to the preponderant monomethyl ethers, which did not separate, as well as a significant amount ofthe dimethyl ethers. The control experiment (no C02) manifested only unreacted PG.
References:
ARCHER DANIELS MIDLAND COMPANY;STENSRUD, Kenneth;VENKITASUBRAMANIAN, Padmesh;MA, Chi-cheng WO2016/99789, 2016, A1 Location in patent:Paragraph 0057; 0058; 0059; 0060
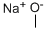
124-41-4
681 suppliers
$12.00/25g
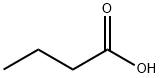
107-92-6
550 suppliers
$9.00/5g

7778-85-0
139 suppliers
$18.00/25g

51729-83-0
17 suppliers
$29.60/1g

105-66-8
145 suppliers
$22.00/25mL

56525-42-9
62 suppliers
$60.00/100mg