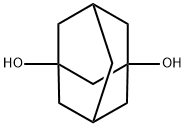
1,3-Adamantanediol synthesis
- Product Name:1,3-Adamantanediol
- CAS Number:5001-18-3
- Molecular formula:C10H16O2
- Molecular Weight:168.23
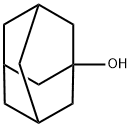
768-95-6
414 suppliers
$10.00/5g

5001-18-3
312 suppliers
$6.00/1g
Yield:5001-18-3 95.1%
Reaction Conditions:
Stage #1:1-adamanthanol with sulfuric acid;acetic anhydride at 14 - 16; for 0.5 h;Large scale;
Stage #2: with nitric acid at 83 - 102;Large scale;Further stages;Temperature;
Steps:
1.1-1.6; 2.1-2.6; 3.1-3.6; 4.1-4.6; 5.1-5.6; 1.1-1.6; 2.1-2.6; 3.1-3.6
(1) Add 75Kg of fuming sulfuric acid with a sulfuric acid mass concentration of 115% in the batching kettle, start stirring, add 2.6Kg of acetic anhydride (13% of the weight of 1-adamantanol), continue stirring for 5 minutes, add purity 20Kg of 99.5% 1-adamantanol crystals, stirred for 0.5 hours at a temperature of 1416 to dissolve into a transparent solution. The starting material weighs 112.6Kg and is marked as material A (adamantanol-smoke Sulfuric acid solution). (2) Use an acid-resistant metering pump to simultaneously pump the prepared starting material A and the pre-filled 9.1Kg 95% nitric acid (marked as material B) in the high tank into the reactor pre-mixing tank, and keep A:B = 112.6:9.1 weight ratio pumping ratio (here the molar ratio of nitric acid to the starting material 1-adamantanol is 1.05:1, and the weight ratio of sulfuric acid to nitric acid is 8.2.:1). After the reaction materials are mixed in the pre-mixing tank, they are immediately pressed into the narrow tube reactor. The materials are heated up during the process of passing through the reactor, and a nitration reaction occurs. The temperature of the water bath outside the reactor is controlled at 8385, and the residence time of the material in the reactor is controlled from 60s70s. It flows out of the reactor and immediately flows into the dilution pre-filled with 50Kg of clear water (here the mass concentration of diluted sulfuric acid is about 68.5%) In the kettle, and constantly stirring, the nitration reaction is terminated. (3) After all the nitrification reaction materials have passed through the reaction tube, heat the nitrification reaction termination material water solution to 100°C102°C, and pump it into the blowing tower while circulating air into the blowing tower, keeping it for 23 When the residual nitric acid content in the water is lower than 0.1%, stop blowing. (4) Transfer the material that has been blown to remove residual nitric acid to the dilution kettle, start stirring, and lower the material liquid to room temperature. Add about 0.6Kg of activated carbon powder (about 3% by weight of 1-adamantanol) to the material, and stir for 5 minutes to allow it to be fully dispersed. Then 70Kg of clean water was slowly flowed from the high tank into the dilution kettle (here the mass concentration of diluted sulfuric acid was about 44.2%), and the stirring was continued for 15 minutes, the stirring was stopped, and the precipitated particles were allowed to stand for 1 hour. The materials are discharged, and most of the acid water is separated by suction filtration, and then the residue is centrifuged to fully remove the residual acid. (5) Transfer the filter residue (mixed particles of nitrification reaction intermediate product and activated carbon) received in the previous step to the alkalization kettle, and add 40Kg of water, start stirring, and then add 20Kg of 35% sodium hydroxide aqueous solution (here sodium hydroxide The molar ratio of the starting material 1-adamantanol is 1.3:1) Slowly flow into the kettle from the high tank, while heating, control the temperature of the materials in the kettle to rise at 95100 (keep it under the premise of not boiling High temperature), heat preservation and reaction for 2 hours, take samples to detect that the hydrolysis reaction in the materials is basically complete (the total amount of residual reaction intermediates is less than 0.1%), cool the materials in the kettle to 2030, and let stand for 12 hours to allow the precipitate Chenghua. The material is discharged, and the filter residue is separated by suction filtration or centrifugation. (6) Stir the filter residue with 90Kg of methanol at 40°C to 45°C for 0.5 hours to dissolve the target product 1,3-adamantanediol. The filter residue (active carbon adsorbed with impurities) is separated by airtight suction filtration, and the filtrate (methanol solution of 1,3-adamantanediol) is transferred to the distillation device. Distillation and concentration, cooling and crystallization, airtight suction filtration to separate the crystals, and drying the crystals at 60°C to obtain the target product 1,3-adamantanediol crystals. 21Kg of 1,3-adamantanediol crystals were obtained, and the crystals were white in appearance. The purity was determined to be 99.5%, and the calculated yield was 95.1% of the theoretical yield (22Kg of pure substance).
References:
CN112250578, 2021, A Location in patent:Paragraph 0053-0118

876-53-9
237 suppliers
$25.00/5g

5001-18-3
312 suppliers
$6.00/1g

281-23-2
470 suppliers
$5.00/25g
700-58-3
448 suppliers
$5.00/5g

768-95-6
414 suppliers
$10.00/5g

5001-18-3
312 suppliers
$6.00/1g

768-95-6
414 suppliers
$10.00/5g

5001-18-3
312 suppliers
$6.00/1g

99181-50-7
101 suppliers
$40.00/250mg