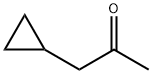
1-CYCLOPROPYL-PROPAN-2-ONE synthesis
- Product Name:1-CYCLOPROPYL-PROPAN-2-ONE
- CAS Number:4160-75-2
- Molecular formula:C6H10O
- Molecular Weight:98.14
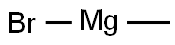
75-16-1
266 suppliers
$12.00/10ml

227322-00-1
31 suppliers
inquiry

4160-75-2
51 suppliers
$175.00/100mg
Yield: 73%
Reaction Conditions:
in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Steps:
1.69.2 Step 2
Into a 500-mL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen, was placed 2-cyclopropyl-N-methoxy-N-methylacetamide (12.0 g, 83.9 mmol, 1.0 equiv), and THF (200 mL).
This was followed by the addition of MeMgBr (3 M in THF) (56.0 mL, 167.8 mmol, 2.0 equiv) dropwise with stirring at 0° C.
The mixture was stirred for 2 h at room temperature.
The reaction was then quenched by the addition of 100 mL of NH4Cl(aq), extracted with 2*100 mL of Et2O, combined with the organic phase, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. 6.0 g (73%) of 1-cyclopropylpropan-2-one was obtained as a yellow oil and used to the next step directly without further purification.
References:
GLOBAL BLOOD THERAPEUTICS, INC.;LI, ZHE;Xu, Qing;Metcalf, Brian Walter;Yee, Calvin Wesley;Rademacher, Peter Michael;Alt, Carsten US2020/190045, 2020, A1 Location in patent:Paragraph 0668; 0669

18729-47-0
14 suppliers
$60.00/5mg

4160-75-2
51 suppliers
$175.00/100mg

917-54-4
174 suppliers
$44.39/50ml

227322-00-1
31 suppliers
inquiry

4160-75-2
51 suppliers
$175.00/100mg

763-13-3
60 suppliers
$51.00/1 g
75-36-5
560 suppliers
$17.92/100G

4160-75-2
51 suppliers
$175.00/100mg

36635-36-6
0 suppliers
inquiry

75-36-5
560 suppliers
$17.92/100G

109-49-9
121 suppliers
$12.00/1g

4160-75-2
51 suppliers
$175.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry