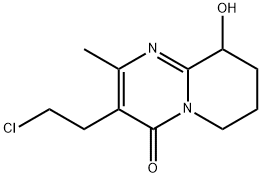
3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one synthesis
- Product Name:3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- CAS Number:130049-82-0
- Molecular formula:C11H15ClN2O2
- Molecular Weight:242.7
![3-(2-Chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one (Paliperidone)](/CAS/GIF/260273-82-3.gif)
260273-82-3
60 suppliers
inquiry
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/GIF/130049-82-0.gif)
130049-82-0
317 suppliers
$55.00/1g
Yield:130049-82-0 99.32%
Reaction Conditions:
Stage #1: 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-onewith hydrogenchloride;palladium 10% on activated carbon in ethanol;water;Green chemistry;
Stage #2: with hydrogen in ethanol;water at 80; under 11251.1 Torr;Catalytic behavior;Green chemistry;Solvent;Reagent/catalyst;Temperature;Pressure;
Steps:
10
1) 200 g of 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-A]pyrimidin-4-one was added to paliperidone hydrogenation precursor. 4L of anhydrous ethanol, 200g of concentrated hydrochloric acid, stirring evenly after adding 5g of 10% Pd / C, fully stirred to form the material I; 2) Transfer the preheated material I and hydrogen from step 1) to the reaction module group of the microchannel reactor. The reaction was carried out, wherein: the flow rate of the slurry pump was adjusted so that the flow rate of the material I was 40.0 g/min, the flow rate of the H2 gas flow meter was adjusted to 700 ml/min, the reaction temperature was 80°C, the temperature of the cooling module was 25°C, and the reaction pressure At 1.5 MPa, the molar ratio of 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-A]pyrimidin-4-one to H2 is 1:3.2. The total reaction time in the reaction module group was 20s; the reaction solution from the cooling module was collected, the catalyst was recovered by filtration, and the solvent was distilled off under reduced pressure. The residue was completely dissolved by adding 500 ml of water. The pH of the saturated NaHCO3 solution was 7 ~8, filtration, add 1.2L of ethyl acetate to the crude product, warm up to 60°C to dissolve, and slowly add 1.2L of n-hexane dropwise to it, add dropwise, cool to 010°C, keep stirring for 1h, filter, filter cake Wash with a small amount of n-hexane and vacuum dry at 50°C for 8 hours to obtain paliperidone intermediate 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl- 4H-pyrido[1,2-a]pyrimidin-4-one 128.91 g, yield 87.32%, purity 99.28%. In order to investigate the recycling efficiency of the catalysts, Pd/C and Pt/C were selected as catalysts. A total of 7 experiments were applied to the cycle. Among them, the amount of catalyst used in each reaction and other key process parameters were the same, and the cycle was examined. The relationship between the catalyst and the reaction conversion rate was applied several times. The results are shown in Tables 1 and 2:
References:
CN108003154,2018,A Location in patent:Page/Page column 7-11
![4H-Pyrido[1,2-a]pyrimidin-4-one, 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-9-(phenylmethoxy)-](/CAS/20210111/GIF/130049-79-5.gif)
130049-79-5
0 suppliers
inquiry
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/GIF/130049-82-0.gif)
130049-82-0
317 suppliers
$55.00/1g
![3-(2-chloroethyl)-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one hydrochloride](/CAS/GIF/849727-62-4.gif)
849727-62-4
32 suppliers
$215.00/25mg
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/GIF/130049-82-0.gif)
130049-82-0
317 suppliers
$55.00/1g
![9-Benxyloxy-3-(2-Chloro ethyl)-2-methyl pyrido[1,2-a]pyrimidine-4-one](/CAS/GIF/147687-17-0.gif)
147687-17-0
106 suppliers
$74.00/5g
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/GIF/130049-82-0.gif)
130049-82-0
317 suppliers
$55.00/1g
![4H-Pyrido[1,2-a]pyrimidine-4,9(6H)-dione, 3-(2-chloroethyl)-7,8-dihydro-2-methyl-, 9-oxime](/CAS/20210305/GIF/1204248-71-4.gif)
1204248-71-4
0 suppliers
inquiry
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/GIF/130049-82-0.gif)
130049-82-0
317 suppliers
$55.00/1g