
2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde synthesis
- Product Name:2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde
- CAS Number:170985-85-0
- Molecular formula:C13H20N2O2
- Molecular Weight:236.31
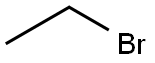
74-96-4
431 suppliers
$9.00/5g
![(S)-[2-(Benzyloxy)propylidene]hydrazinecarboxaldehyde](/CAS2/GIF/170985-84-9.gif)
170985-84-9
51 suppliers
$125.00/500mg
![2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](/CAS/GIF/170985-85-0.gif)
170985-85-0
114 suppliers
inquiry
Yield:170985-85-0 66%
Reaction Conditions:
Stage #1: ethyl bromidewith iodine;magnesium in tert-butyl methyl ether at 40 - 55; for 2 h;Inert atmosphere;
Stage #2: (S)-N'-(2-(benzyloxy)propylidene)formylhydrazidewith N,O-bis-(trimethylsilyl)-acetamide in tert-butyl methyl ether at 5 - 30; for 9 h;Inert atmosphere;Temperature;
Steps:
1.4; 2.4
4. 20 g (0.83 mol) of magnesium strip and 0.0325 g (0.13 mmol) of iodine.150 ml of methyl tert-butyl ether was added to a 500 ml three-neck round bottom flask, protected with nitrogen.After the mixture was heated to 40 ° C, 90.53 g (0.83 mol) of ethyl bromide was slowly added dropwise.After the addition was completed, 60 ml of methyl tert-butyl ether was added.The reaction mixture was then warmed to 55 ° C and the reaction was stirred for 2 hours;After the reaction is completed, the temperature is lowered to 5 ° C to obtain a reaction mixture containing a Grignard reagent;Further, 81.2 g (0.4 mol) of N,O-bistrimethylsilylacetamide was slowly added to dissolve (S)-N'-(2-benzyloxypropylene)formylhydrazide 40 g (0.2 mol). Of 150 ml of methyl tert-butyl ether,The reaction was stirred at 25 to 30 ° C for 1 hour, and the resulting solution was protected with nitrogen.Added to the above Grignard reaction mixture at 5 ° C,After the addition was completed, the reaction mixture was heated to 25 to 30 ° C and stirred for 8 hours.After the reaction was completed, a concentration of 8% acetic acid solution was added to the reaction mixture at 0 ° C.After stirring for 30 minutes, the organic layer was separated, washed once with saturated brine, and then washed once.Dry over anhydrous sodium sulfate and filter.Removal of methyl tert-butyl ether gave the product N'-[(2S,3S)-2-(benzyloxy)pentan-3-yl]carbonylhydrazide 31.17 g (0.132 mol), yield 66%.
References:
CN109796368,2019,A Location in patent:Paragraph 0016; 0039; 0043; 0044; 0048; 0052; 0053

81445-44-5
44 suppliers
$125.00/25mg
![2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](/CAS/GIF/170985-85-0.gif)
170985-85-0
114 suppliers
inquiry
![1-[(2S)-2-(benzyloxy)propanoyl]pyrrolidine](/CAS/20150408/GIF/122151-32-0.gif)
122151-32-0
42 suppliers
inquiry
![2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](/CAS/GIF/170985-85-0.gif)
170985-85-0
114 suppliers
inquiry

33106-32-0
44 suppliers
inquiry
![2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](/CAS/GIF/170985-85-0.gif)
170985-85-0
114 suppliers
inquiry

406940-46-3
0 suppliers
inquiry
![2-[(1S,2S)-1-Ethyl-2-(phenylmethoxy)propyl]hydrazinecarboxaldehyde](/CAS/GIF/170985-85-0.gif)
170985-85-0
114 suppliers
inquiry