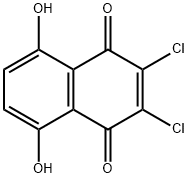
2,3-DICHLORO-5,8-DIHYDROXY-1,4-NAPHTHOQUINONE synthesis
- Product Name:2,3-DICHLORO-5,8-DIHYDROXY-1,4-NAPHTHOQUINONE
- CAS Number:14918-69-5
- Molecular formula:C10H4Cl2O4
- Molecular Weight:259.04

1122-17-4
138 suppliers
$7.00/1g
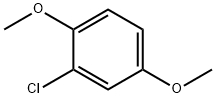
2100-42-7
120 suppliers
$10.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14918-69-5
70 suppliers
$31.00/100mg

15012-64-3
0 suppliers
inquiry
Yield: 54% , 1% , 7%
Reaction Conditions:
with aluminum (III) chloride;sodium chloride in melt at 150 - 175; for 0.116667 h;Friedel-Crafts Acylation;Time;
Steps:
Cycloacylation of 2-chlorohydroquinone dimethyl ether (7).
General procedure: A mixture of substrate 7 (1.38 g, 8.0 mmol) and anhydride 9 (2.67 g, 16.0 mmol) was added to the melt of anhydrous AlCl3 (8.54 g, 64.0 mmol) and NaCl (1.87 g, 32.0 mmol) with vigorous stirring at 150 °C, the temperature of the mixture was increased to the values given in Table 1 and maintained for the indicated time. Then, the reaction mixture was cooled to room temperature, diluted with 10% aqueous HCl (50 mL), and was allowed to stand for 12 h. The product formed was separated, washed with hot (55-60 °C) water (10×40 mL), dried to the constant weight, and subjected to chromatography on a column with SiO2. The elution with the mixture of hexane-benzene (10 : 1)gave 2,3,6,7tetrachloro5,8dihydroxy1,4naphthoquinone (2)(0.013-0.079 g, 0.5-3%), Rf 0.65 (benzene-hexane (4 : 1)), redneedles, m.p. 256-258 °C (Ref. 45: 258 °C). IR (CDCl3), ν/cm-1:3400-2250 (αOH), 1627 (C=O, C=C), 1568 (C=C), 1405.1H NMR (CDCl3), δ: 12.88 (s, 2 H, 2 αOH). 13C NMR (CDCl3),δ: 109.2 (C(4a), C(8a)); 139.1 (C(2), C(3), C(6), C(7)); 167.2(C(1), C(4), C(5), C(8)). MS, m/z (Irel (%)): 327/329/331/333/335 [M + 1]+ (58), 326/328/330/332/334 [M]+ (100), 292/294/296/298 [M - Cl + 1]+ (20), 291/293/295/297 [M - Cl]+ (61),257/259//261 [M - 2Cl + 1]+ (5), 256/258//260 [M - 2Cl]+ (17).The elution with the mixture of hexane-benzene (4 : 1) gave2,3,6trichloro5,8dihydroxy1,4naphthoquinone (1) (1.01-1.43 g,43-61%), Rf 0.48 (benzene-hexane (4 : 1)), red needles,m.p. 174-176 °C (Ref. 24: 174-176 °C). IR (CDCl3), ν/cm-1:3350-2200 (αOH), 1628 (C=O, C=C), 1566 (C=C), 1493,1401. 1H NMR (CDCl3), δ: 7.45 (s, 1 H, H(7)); 12.31 (s, 1 H,C(8) OH); 12.74 (s, 1 H, C(5) OH). 13C NMR (CDCl3), δ: 109.6(C(8a)); 110.8 (C(4a)); 130.6 (C(7)); 137.5 (C(6)); 141.5 (C(3));142.5 (C(2)); 159.6 (C(5)); 162.8 (C(8)); 174.4 (C(1)); 175.2(C(4)). MS, m/z (Irel (%)): 293/295/297/299 [M + 1]+ (13), 292/294/296/298 [M]+ (100), 258/260/262 [M - Cl + 1]+ (11), 257/259/261 [M - Cl]+ (14), 223/225 [M - 2Cl + 1]+ (15), 222/224[M - 2Cl]+ (18).The elution with benzene gave 2,3dichloro5,8dihydroxy1,4naphthoquinone (10) (0.124-0.207 g, 6-10%), Rf 0.30(benzene-hexane (4 : 1)), red needles, m.p. 194-196 °C (from1,4dioxane) (Ref. 10: 192 °C, Ref. 18: 198-199 °C). IR(CDCl3), ν/cm-1: 3400-2250 (αOH), 1625 (C=O, C=C), 1571(C=C), 1403. 1H NMR (CDCl3), δ: 7.33 (s, 2 H, H(6), H(7));12.34 (s, 2 H, 2 αOH). 13C NMR (CDCl3), δ: 110.4 (C(4a),C(8a)); 131.1 (C(6), (C(7)); 142.9 (C(2), (C(3)); 161.1 (C(5),(C(8)); 177.2 (C(1), (C(4)). MS, m/z (Irel (%)): 259/261/263[M + 1]+ (59), 258/260/262 [M]+ (100), 257/259/261 [M - 1]+(45), 224/226 [M - Cl + 1]+ (19), 223/225 [M - Cl]+ (22), 222/224 [M - Cl - 1]+ (17).The elution with the mixture of benzene-acetone (1 : 1)gave 2,5dichloro4,7dihydroxy3hydroxycarbonylinden1one(11) (0.024-0.065 g, 1.5-4%), Rf 0.50 (hexane-acetone (1 : 1))reddish yellow needles, m.p. >350 °C. IR (KBr), ν/cm-1: 3377(OH), 3214 (OH), 3280-2150 (COOH), 2923, 2853, 2361, 1698(C=O), 1681 (C=O), 1619 (C=C), 1572, 1438, 1385, 1302, 1288,1240, 1181, 1163, 1143, 1047, 1032, 881, 777, 743. 1H NMR(DMSOd6), δ: 6.02 (br.s, 1 H, C(4) OH); 6.95 (s, 1 H, H(6));10.77 (br.s, 1 H, C(3) COOH); 16.00 (s, 1 H, C(7) OH). 13C NMR(DMSOd6), δ: 111.3 (C(7a)); 122.7 (C(6)); 123.8 (C(3a)); 131.8(C(2)); 134.1 (C(5)); 142.8 (C(3)); 143.0 (C(4)); 151.0 (C(7));166.9 (COOH); 185.3 (C(1)). MS (EI, 15 eV), m/z (Irel (%)):275/277/279 [M + 1]+ (31), 274/276/278 [M]+ (100), 273/275/277 [M - 1]+ (26). Found (%): C, 43.76; H, 1.50; Cl, 25.53.C10H4Cl2O5. Calculated (%): C, 43.67; H, 1.47; Cl, 25.78.
References:
Novikov;Balaneva;Shestak;Anufriev, V. Ph.;Glazunov [Russian Chemical Bulletin,2016,vol. 65,# 4,p. 993 - 1003][Izv. Akad. Nauk, Ser. Khim.,2016,# 4,p. 993 - 1003,11]

1122-17-4
138 suppliers
$7.00/1g
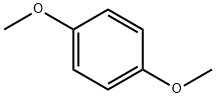
150-78-7
510 suppliers
$5.00/25g

14918-69-5
70 suppliers
$31.00/100mg

1122-17-4
138 suppliers
$7.00/1g

2100-42-7
120 suppliers
$10.00/5g

608-44-6
2 suppliers
inquiry
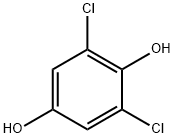
20103-10-0
36 suppliers
$105.00/50 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

824-69-1
59 suppliers
$41.79/1g

14918-69-5
70 suppliers
$31.00/100mg

15012-64-3
0 suppliers
inquiry
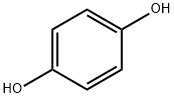
123-31-9
825 suppliers
$13.00/25g
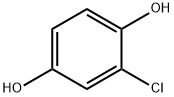
615-67-8
177 suppliers
$13.00/5g

78456-63-0
2 suppliers
inquiry

14918-69-5
70 suppliers
$31.00/100mg
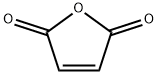
108-31-6
835 suppliers
$13.47/50 G

615-67-8
177 suppliers
$13.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14918-69-5
70 suppliers
$31.00/100mg
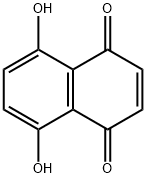
475-38-7
130 suppliers
$65.00/250 mg