
2,6-DIIODO-4-NITROANILINE synthesis
- Product Name:2,6-DIIODO-4-NITROANILINE
- CAS Number:5398-27-6
- Molecular formula:C6H4I2N2O2
- Molecular Weight:389.92
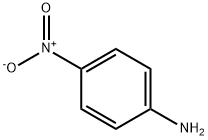
100-01-6
10 suppliers
$11.19/25G

5398-27-6
86 suppliers
$62.00/5G
Yield: 92%
Reaction Conditions:
with N-iodo-succinimide in water;acetonitrile at 25 - 30; for 0.25 h;
Steps:
General procedure for the diiodination reaction
To a solution of 4-nitroaniline, 1a (1.0 g, 7.2 mmol) and 0.01 mL water, sulfated polyborate (0.025 g, 2.5 wt%) in 7 mL anhyd. MeCN in a round bottom flask was added NIS solution (3.26 g, 14.4 mmol in 4.0 mL anhyd. MeCN) dropwise. The reaction mixture was stirred for 15 minutes at 25-30 °C, monitored by GC. After completion of the reaction, MeCN was distilled under vacuum at 40-45 °C. The residue was treated with 10 mL each of MDC and water, stirred for the mixture for 10-15 minutes; MDC layer was separated, washed with 1% sodium thiosulphate, dried over sodium sulfate, and evaporated under vacuum to obtain 2,6-diiodo-4-nitroaniline, 3a, 2.8 g (92% yield, 99% purity) as yellow crystalline solid. m.p. 250-252 °C (lit.51 105-109 °C); 1H NMR (400 MHz, DMSO-d6) δ 8.40 (s, 2H), 6.30 (s, 2H); C6H4I2N2O2; 389.92; GC-MS m/z 390.0. All the products of diiodination were identified by 1H NMR and GC-MS or LC-MS.
References:
Chaturbhuj, Ganesh;Ganwir, Prerna;Misal, Balu;Palav, Amey [Tetrahedron Letters,2021,vol. 74,art. no. 153154] Location in patent:supporting information
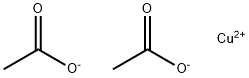
142-71-2
424 suppliers
$5.00/10g
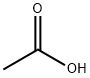
64-19-7
1530 suppliers
$10.00/25ML

100-01-6
10 suppliers
$11.19/25G

6293-83-0
155 suppliers
$6.00/250mg

5398-27-6
86 suppliers
$62.00/5G

19591-18-5
1 suppliers
inquiry
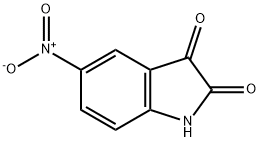
611-09-6
184 suppliers
$6.00/1g

5398-27-6
86 suppliers
$62.00/5G

100-01-6
10 suppliers
$11.19/25G

6293-83-0
155 suppliers
$6.00/250mg

5398-27-6
86 suppliers
$62.00/5G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5398-27-6
86 suppliers
$62.00/5G