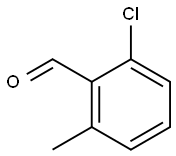
2-CHLORO-6-METHYLBENZALDEHYDE synthesis
- Product Name:2-CHLORO-6-METHYLBENZALDEHYDE
- CAS Number:1194-64-5
- Molecular formula:C8H7ClO
- Molecular Weight:154.59
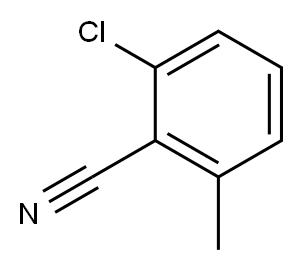
6575-09-3
92 suppliers
$26.39/1g

1194-64-5
109 suppliers
$17.00/250mg
Yield: 23%
Reaction Conditions:
Stage #1:2-chloro-6-methyl-benzonitrile with diisobutylaluminium hydride in tetrahydrofuran at 45;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; pH=3 at 0;
Steps:
32.Ex-32A
Example 32: 4-[l-(2-Chloro-6-methyl-benzoyl)-vinyl]-3-thien-2-yl-benzoic acid[0341] Ex-32A: In a 100 mL round-bottom flask 2-chloro-6-methyl-benzonitrile (2.01 g, 13.3 mmol) was combined with THF (28 mL). Di-isobutyl aluminum hydride (1 M in THF, 14.6 mL) was added quickly dropwise. After addition was complete, the reaction was heated to 45 0C overnight, at which point HPLC indicated only 25% conversion. Additional DIBAL-H (6.7 mL) was added and the reaction was heated at 45 0C for another 2 h, at which point HPLC indicated the reaction was still incomplete while impurities were forming. The reaction was cooled in an ice bath and MeOH was added, followed by H2O, then 1 N HCl until the pH was ~3. The solution was extracted with EtOAc. The organic phase was washed with 2 N Na2CO3, then brine, dried over Na2SO4, filtered, concentrated, and dried. The crude material was purified by silica gel chromatography (2.5% EtOAc in hexanes) to provide 0.48 g (23%) of 2-chloro-6-methyl- benzaldehyde as a faint yellow oil. 1H-NMR (CDCl3) δ 10.66 (s, IH), 7.26-7.39 (m, 2H), 7.16 (d, J= 7.2 Hz, IH), 2.59 (s, 3H).
References:
ATHEROGENICS, INC. WO2006/4903, 2006, A2 Location in patent:Page/Page column 124-125

529-20-4
325 suppliers
$5.00/5g

1194-64-5
109 suppliers
$17.00/250mg

77206-89-4
20 suppliers
inquiry

1194-64-5
109 suppliers
$17.00/250mg
35490-90-5
55 suppliers
$21.00/100mg
676-58-4
276 suppliers
$15.00/25ml

1194-64-5
109 suppliers
$17.00/250mg

387-45-1
363 suppliers
$9.00/1g

1194-64-5
109 suppliers
$17.00/250mg