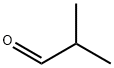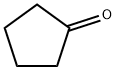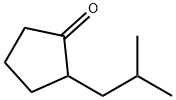
2-Iso-butylcyclopentanone synthesis
- Product Name:2-Iso-butylcyclopentanone
- CAS Number:4668-65-9
- Molecular formula:C9H16O
- Molecular Weight:140.22
Yield:4668-65-9 65 %Chromat.
Reaction Conditions:
with hydrogen at 140; under 26252.6 Torr; for 6.66667 h;Autoclave;
Steps:
2.4. One-pot synthesis of 2-alkyl cycloketones from aldehydes andcycloketones
General procedure: Take 2BCH as example, 18 g (0.25 mol) n-butanal, 54 g (0.55 mol)cyclohexanone, and 3.7 g catalysts were added into a 250 mL autoclave.Oxygen in autoclave was displaced thrice by 0.2 MPa N2 and 0.5 MPa H2in sequence. Then, 3.5 MPa H2 was charged into the autoclave at roomtemperature. The reaction was carried out at 140 C for 400 min understirring at 400 rpm followed by cooling to room temperature naturally.The other 2-alkyl cycloketones were synthesized with the same cycloketone/aldehyde molar ratio.
References:
Xue, Weiyang;Gu, Bin;Wu, Huiling;Liu, Mengyang;He, Songbo;Li, Jingmei;Rong, Xin;Sun, Chenglin [Applied Catalysis A: General,2021,vol. 616,art. no. 118107]

16856-72-7
0 suppliers
inquiry

4668-65-9
9 suppliers
inquiry

56475-43-5
0 suppliers
inquiry

4668-65-9
9 suppliers
inquiry
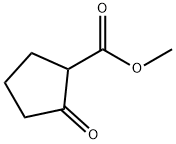
10472-24-9
453 suppliers
$6.00/10g

4668-65-9
9 suppliers
inquiry

471256-87-8
0 suppliers
inquiry

4668-65-9
9 suppliers
inquiry