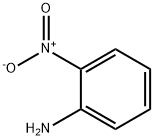
2-Nitroaniline synthesis
- Product Name:2-Nitroaniline
- CAS Number:88-74-4
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12

616-84-2
121 suppliers
inquiry

88-74-4
56 suppliers
$10.00/1g
Yield:88-74-4 91%
Reaction Conditions:
with phosphoric acid in sulfolane at 120;Reagent/catalyst;Solvent;Temperature;
Steps:
1-23 Example 5
Example 5A method for preparing 2-nitro-aniline specifically includes the following steps:(1) Add the substrate 2-nitro-4-sulfonic aniline to a high boiling point and high polarity solvent for dissolution, then add an acid, control the temperature to 120°C for desulfonic acid reaction, and HPLC monitor after the reaction is over , The resulting reaction solution is controlled under reduced pressure and concentrated at 80-90°C and a vacuum of 5-10mmHg to remove the high-boiling high-polarity solvent to obtain a concentrate, and the recovered high-boiling high-polarity solvent is reused;The high-boiling point and high-polarity solvent is sulfolane, and its dosage is calculated according to the ratio of 2-nitro-4-sulfonic aniline: 1g:5mL of the high-polarity and high-boiling point solvent;The acid is phosphoric acid, and its dosage is calculated based on the molar ratio of 2-nitro-4-sulfonic aniline: acid of 1:1.2;(2) Add water to the concentrate obtained in step (1) and mix well, adjust the pH to 8 with a base, and then use ethyl acetate (the amount of ethyl acetate required for each extraction to prepare step (1) The used 2-nitro-4-sulfonic aniline is calculated, that is 2-nitro-4-sulfonic aniline: ethyl acetate is 1g: 3mL) extract 3 times, combine the organic phases obtained 3 times, and then saturated The alkaline aqueous solution is washed to 8, and then the temperature is controlled at 40-45°C for concentration and drying to obtain a crude product with a yield of 91% and a purity (HPLC) greater than 97%.The amount added to the above concentrate is calculated based on the 2-nitro-4-sulfonic acid aniline used in the preparation of the concentrate in step (1), that is, 2-nitro-4-sulfonic acid aniline: water is 1g: 3mL The alkali is sodium hydroxide;The saturated aqueous alkali solution is saturated sodium hydroxide aqueous solution.The crude product obtained above was verified by the hydrogen NMR spectrum, and the result showed that it was the expected 2-nitro-aniline product.The aqueous layer extracted by the organic solvent in the above preparation process is collected and concentrated to obtain sodium sulfate of waste salt. After drying, it is calculated as tons of 2-nitro-aniline to prepare per ton of 2-nitro-aniline, and finally 3.3 tons of waste salt are produced.
References:
Shanghai Wanxiang Pharmaceutical Co., Ltd.;Yuan Xiangfu CN111848410, 2020, A Location in patent:Paragraph 0014-0068

577-19-5
25 suppliers
$14.00/5g

88-74-4
56 suppliers
$10.00/1g
88-73-3
49 suppliers
$16.00/25g

88-74-4
56 suppliers
$10.00/1g
95-54-5
545 suppliers
$9.00/1g

88-74-4
56 suppliers
$10.00/1g
609-73-4
15 suppliers
$10.00/5g

88-74-4
56 suppliers
$10.00/1g