2-Phenylquinolin-4-ol synthesis
- Product Name:2-Phenylquinolin-4-ol
- CAS Number:1144-20-3
- Molecular formula:C15H11NO
- Molecular Weight:221.25
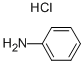
142-04-1
179 suppliers
$10.00/10g
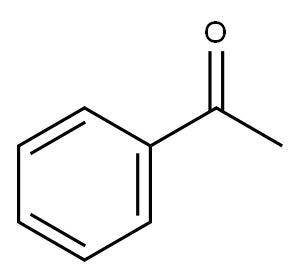
94-02-0
387 suppliers
$14.00/5g

1144-20-3
50 suppliers
$60.00/100mg
Yield:-
Reaction Conditions:
with PPA;aniline in ammonium hydroxide;water;ethyl acetate;toluene
Steps:
1.B Synthesis of 2-phenyl-4-hydroxyquinoline (1b):
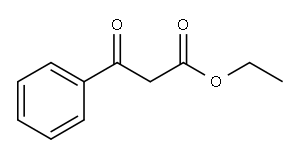
Example 1B Synthesis of 2-phenyl-4-hydroxyquinoline (1b): Commercially available ethyl benzoylacetate (6.00 g, 31.2 mmol) was heated at 85° C. (sealed tube) in 75 mL of 30% NH4OH for 2 hours. The solid formed upon cooling was filtered and refluxed in water for 2 hours. The solution was extracted three times with CH2Cl2. The organic layers were combined, dried over MgSO4, filtered and concentrated. The yellow residue was flash chromatographed on silica gel, eluding with EtOAc:hexane (3:7), to give the corresponding amide as a white solid, 1.60 g, 31% yield. This amide (250 mg, 1.53 mmol) was refluxed using a Dean-Stark apparatus with aniline (143 mg, 1.53 mmol) and aniline.HCl (10 mg, 0.08 mmol) in toluene (10 mL) for 16 h. The solution was concentrated to afford a brown oil that was mixed with polyphosphoric acid (2 g) and heated at 135° C. for 20 min. The reaction mixture was poured into water and adjusted to pH 8 with 5 M NaOH. The aqueous suspension was extracted twice with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated. The residue was flash chromatographed on silica gel, eluding with 3% MeOH in ethyl acetate, to give 2-phenyl-4-hydroxyquinoline (2), 67 mg, 20% yield. 1H NMR (DMSO-d6) δ 8.11 (d, J=7 Hz, 1H), 7.86-7.83 (m, 2H), 7.77 (d, J=8 Hz, 1H), 7.68 (dd, J=8, 7 Hz, 1H), 7.61-7.58 (m, 3H), 7.35 (dd, J=8, 7 Hz, 1H), 6.34 (s, 1H).
References:
Boehringer Ingelheim (Canada) Ltd. US6767991, 2004, B1

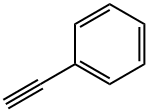
16619-14-0
33 suppliers
$45.00/50mg

1144-20-3
50 suppliers
$60.00/100mg

5855-52-7
22 suppliers
$45.00/100mg

1144-20-3
50 suppliers
$60.00/100mg