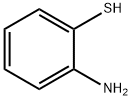
2-(Phenylthio)aniline synthesis
- Product Name:2-(Phenylthio)aniline
- CAS Number:1134-94-7
- Molecular formula:C12H11NS
- Molecular Weight:201.29
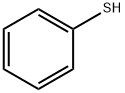
95-16-9
393 suppliers
$14.14/10gm:
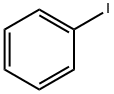
591-50-4
471 suppliers
$10.00/1g

1134-94-7
248 suppliers
$7.00/5g
Yield:1134-94-7 99%
Reaction Conditions:
with tetra(n-butyl)ammonium hydroxide;water;copper(l) chloride at 50; for 12 h;Inert atmosphere;
Steps:
General procedure for C-S coupling catalyzed by CuCl (procedure A):
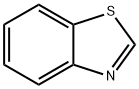
General procedure: After standard cycles of evacuation and back-filling with dry and pure argon, an oven-dried Schlenk tube equipped with a magnetic stirring bar was charged with CuCl (5 mol%), the aryl iodides if a solid (1.2 equiv). The tube was evacuated and backfilled with argon (this procedure was repeated three times). Under a counter flow of argon, benzothiazole (0.5 mmol), aryl iodides if a liquid (1.2 equiv) and degassed 40% tetra-n-butylammonium hydroxides water solution (1.0 mL, 3.0 equiv) were added by syringe. The tube was sealed and the mixture was allowed to stir at 50 oC for 12 h. The reaction mixture was then allowed to cool to ambient temperature. Then, the mixture was quenched by the addition of a saturated NH4Cl solution (1.5 mL) and extracted with ethyl acetate (3×10 mL). Organic layers were gathered, dried over Na2SO4, filtered and concentrated in vacuum to yield the crude product. The obtained crude was purified by column chromatography on silica gel and the product was dried under vacuum for at least 0.5 h.
References:
Feng, Yi-Si;Qi, Hong-Xia;Wang, Wei-Cheng;Liang, Yu-Feng;Xu, Hua-Jian [Tetrahedron Letters,2012,vol. 53,# 23,p. 2914 - 2917] Location in patent:supporting information; experimental part
4171-83-9
152 suppliers
$21.00/250mg

1134-94-7
248 suppliers
$7.00/5g