
3,5-DI-TERT-BUTYL-4-HYDROXYACETOPHENONE synthesis
- Product Name:3,5-DI-TERT-BUTYL-4-HYDROXYACETOPHENONE
- CAS Number:14035-33-7
- Molecular formula:C16H24O2
- Molecular Weight:248.36
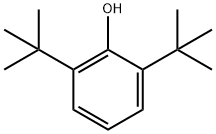
128-39-2
341 suppliers
$5.00/25g
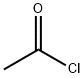
75-36-5
559 suppliers
$17.92/100G

14035-33-7
53 suppliers
$18.00/250mg
Yield: 95%
Reaction Conditions:
AlCl3 in tetrachloromethane
Steps:
1 EXAMPLE 1
EXAMPLE 1 Eight hundred milliliters of dry CCl4 was cooled to 0° to 5° C. Anhydrous AlCl3, 96.4 grams (0.70 mole) was added followed by the dropwise addition of 50 milliliters (0.70 mole) of acetyl chloride. After the addition of acetyl chloride was completed, 103 grams (0.50 mole) of 2,6-di-t-butylphenol dissolved in 100 milliliters of CCl4 was added over a 1 hour period while maintaining a temperature below 10° C. The mixture was slowly warmed to room temperature and stirred an additional hour. The aluminum chloride/3,5-di-t-butyl-4-hydroxyacetophenone complex was hydrolyzed by pouring the reaction mixture into an excess of cold dilute hydrochloric acid. The lower CCl4 layer was separated, and the solvent was evaporated to obtain 118 grams of 3,5-di-t-butyl-4-hydroxyacetophenone with a 95 percent yield and a melting point of 146 to 148° C.
References:
The Goodyear Tire & Rubber Company US4072724, 1978, A

128-39-2
341 suppliers
$5.00/25g

14035-33-7
53 suppliers
$18.00/250mg

19263-37-7
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14035-33-7
53 suppliers
$18.00/250mg

128-39-2
341 suppliers
$5.00/25g
64-19-7
1529 suppliers
$10.00/25ML

14035-33-7
53 suppliers
$18.00/250mg
108-22-5
244 suppliers
$19.00/25mL

128-39-2
341 suppliers
$5.00/25g

14035-33-7
53 suppliers
$18.00/250mg