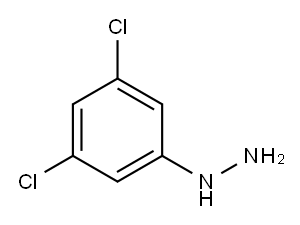
3,5-DICHLOROPHENYLHYDRAZINE synthesis
- Product Name:3,5-DICHLOROPHENYLHYDRAZINE
- CAS Number:39943-56-1
- Molecular formula:C6H6Cl2N2
- Molecular Weight:177.03
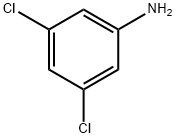
626-43-7
314 suppliers
$10.00/1g

39943-56-1
63 suppliers
$15.00/500mg
Yield: 68.6%
Reaction Conditions:
Stage #1:3,5-Dichloroaniline with hydrogenchloride;sodium nitrite in water at -10; for 0.5 h;
Stage #2: with hydrogenchloride;tin(ll) chloride in water at 4; for 7 h;
Steps:
Step 1: Preparation of Intermediate 3,5-chlorophenyl)hydrazine (I-la)
Step 1: Preparation of Intermediate 3,5-chlorophenyl)hydrazine (I-la) To a cold suspension of 3, 5-dichloroaniline (15.0 g, 92.58 mmol, x 4 batches) in conc. HCI (45 mL, for each batch) was added a solution of NaNO2 (7 g, 101 .44 mmol, for each batch) in water (65 mL, for each batch) at 100C and stirred for 30 minutes. Then a solution of SnCI2 (52.49 g, 277.7 mmol, for each batch) in conc. HCI (45 mL, foreach batch) was added slowly. After addition was complete, a white precipitate was formed which was stored at 4°C for 7 h. The precipitated solid was collected by filtration, washed with hexane and suspended in 10% aq NaOH solution (pH = 13) and extracted with Ethyl acetate (4 x 200 mL). The organic layer was washed with water (250 mL), brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. Thesolids thus obtained from 4 batches were combined and was washed with n-hexane to afford 45 g (68.6%) of 3,5-chlorophenyl)hydrazine a-ia) as an off-white solid.NMR (400 MHz, DMSO-d6): 66.75 (5, 1 H), 6.72 (5, 2H), 5.30 (br s, 1 H, -NH), 3.58 (br s, 2H, -NH2). ESI MS: m/z 179.0 (M+2H).
References:
NOVARTIS AG;JIRICEK, Jan;KONDREDDI, Ravinder Reddy;SMITH, Paul William WO2014/37900, 2014, A1 Location in patent:Page/Page column 26

108-70-3
242 suppliers
$19.89/442235

39943-56-1
63 suppliers
$15.00/500mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

39943-56-1
63 suppliers
$15.00/500mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
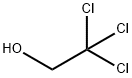
115-20-8
279 suppliers
$8.00/25g

39943-56-1
63 suppliers
$15.00/500mg