
3-Amino-4-phenylbutyric acid hydrochloride synthesis
- Product Name:3-Amino-4-phenylbutyric acid hydrochloride
- CAS Number:3060-41-1
- Molecular formula:C10H14ClNO2
- Molecular Weight:215.68
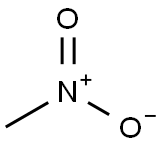
75-52-5
4 suppliers
$35.28/100g
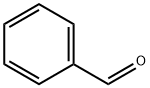
100-52-7
947 suppliers
$20.37/250g

105-53-3
727 suppliers
$5.00/25g

3060-41-1
141 suppliers
$10.00/1g
Yield:3060-41-1 127 g
Reaction Conditions:
Stage #1: nitromethane;benzaldehyde in ethanol at -5;
Stage #2: with sodium methylate in ethanol; for 2 h;
Stage #3: diethyl malonateFurther stages;Reagent/catalyst;
Steps:
1-3 Example 3
In this embodiment, a one-pot method for preparing 4-amino-3-phenylbutyrate hydrochloride, the steps are as follows:(1) Condensation reactionUsing 262g benzaldehyde and 151g nitromethane as reactant I, slowly add 1000mL ethanol at -5 to obtain reactant I ethanol solution, then add dropwise 500g 30% (w/w) sodium methoxide ethanol solution, and stir to react 2h; After the reaction is complete, add 400 mL of 30% (w/w) hydrochloric acid ethanol solution to neutralize to pH=2 to obtain Intermediate I reaction solution;(2) Addition reactionUsing 240g diethyl malonate and 125g sodium methoxide as reactant II, slowly add 1000mL ethanol at -5°C to obtain reactant II ethanol solution. Add the above-mentioned intermediate I reaction solution dropwise with stirring, and the addition is complete After reacting for 2 hours, 300 mL of 30% (w/w) hydrochloric acid ethanol solution was added to neutralize to pH=2 to obtain Intermediate II reaction liquid;(3) Hydrogenation reactionTransfer the above-mentioned Intermediate II reaction solution to a hydrogenation kettle, add 7g of palladium-carbon catalyst, add hydrogen gas at 55°C, and react for 8 hours to obtain Intermediate III reaction solution; Concentrate the Intermediate III reaction solution to the original volume 1/4, cooled to 0 for crystallization, separated by filtration to obtain 446g intermediate III precipitate and 950mL mother liquor I; the above 446g intermediate III solids were washed with water to obtain 177g intermediate III fine products; mother liquor I was applied;(4) Hydrolysis reactionPut the above-mentioned 177g of intermediate product III into the hydrolysis kettle, add 270g of 30% (w/w) hydrochloric acid, and react at 95°C for 24 hours to obtain 4-amino-3-phenylbutyrate hydrochloride reaction solution; Activated carbon was added to the 4-amino-3-phenylbutyrate hydrochloride reaction solution for decolorization, filtered to remove the activated carbon, the filtrate was cooled to 0°C for crystallization, and separated by filtration to obtain 141g 4-amino-3-phenylbutyrate hydrochloride acid Salt precipitation and 192mL mother liquor II; the above 141g 4-amino-3-phenylbutyrate hydrochloride solid is dried to obtain 127g 4-amino-3-phenylbutyrate hydrochloride finished product; the mother liquor II is used.
References:
CN111393312,2020,A Location in patent:Paragraph 0031-0060

1198-97-6
125 suppliers
$21.00/250mg

3060-41-1
141 suppliers
$10.00/1g

62384-61-6
0 suppliers
inquiry

3060-41-1
141 suppliers
$10.00/1g

84872-79-7
5 suppliers
inquiry

3060-41-1
141 suppliers
$10.00/1g

4160-80-9
11 suppliers
$45.00/2.5mg

3060-41-1
141 suppliers
$10.00/1g