
4-(3-Methyloxetan-3-yl)benzoic acid synthesis
- Product Name:4-(3-Methyloxetan-3-yl)benzoic acid
- CAS Number:1315567-78-2
- Molecular formula:C11H12O3
- Molecular Weight:192.21
872882-97-8
43 suppliers
$65.00/10mg
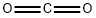
124-38-9
122 suppliers
$175.00/23402

1315567-78-2
25 suppliers
$200.00/50mg
Yield: 51%
Reaction Conditions:
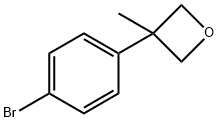
Stage #1:3-(4-bromophenyl)-3-methyloxetane with n-butyllithium in tetrahydrofuran;hexane at -78; for 1.16667 h;Cooling with acetone-dry ice;
Stage #2:carbon dioxide in tetrahydrofuran;hexane
Steps:
I-25 Step 1: 4-(3-Methyl-oxetan-3-yl)-benzoic acid
In a 250 mL three-necked flask, 3-(4-bromophenyl)-3-methyloxetane (1.05 g, 4.62 mmol) was combined with THF (35 mL) to give a colorless solution. This solution was cooled to -78 °C in a dry ice-acetone bath. To the cold solution was added drop wise a 1.6 M solution of nBuLi in hexanes (3.32 mL, 5.32 mmol). Drop wise addition occurred over the course of 10 minutes. The reaction mixture was stirred at -78 °C for 1 hour. After this time, carbon dioxide gas, which was generated in a separate flask from dry ice, was added to the reaction mixture via a long needle. The reaction mixture quickly turned to light yellow. Carbon dioxide was bubbled in at low temperature for another 20 minutes. After this time, the reaction mixture was a white suspension. The reaction mixture was warmed to room temperature then slowly quenched with water. The organic solvent was evaporated off. The resulting mixture was extracted with a 1: 1 solution of ethyl acetate and hexanes. The aqueous phase was then brought to acidic pH though the addition of 4 N aqueous HC1. The resulting white suspension was vacuum filtered using a Biichner funnel. The collected white solids were further dried down on the vacuum funnel and then further dried in the vacuum oven, giving 4-(3-methyl-oxetan-3-yl)-benzoic acid (456 mg, 51%). 1H NMR (300 MHz, DMSO-6) δ ppm 12.89 (br. s., 1 H), 7.93 (d, J = 8.48 Hz, 2 H), 7.36 (d, J = 8.67 Hz, 2 H), 4.81 (d, J = 5.84 Hz, 2 H), 4.56 (d, J = 6.03 Hz, 2 H), 1.64 (s, 3 H).
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;BHAGIRATH, Niala;DOMINIQUE, Romyr;KENNEDY-SMITH, Joshua;LOPEZ-TAPIA, Francisco Javier;MERTZ, Eric;QIAO, Qi;SO, Sung-Sau WO2014/64131, 2014, A2 Location in patent:Page/Page column 97; 98