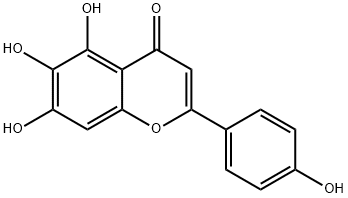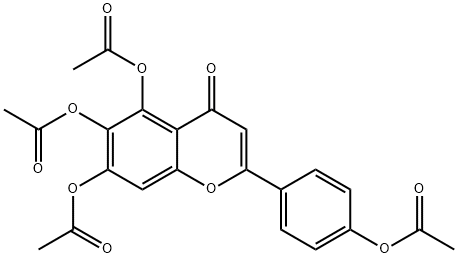
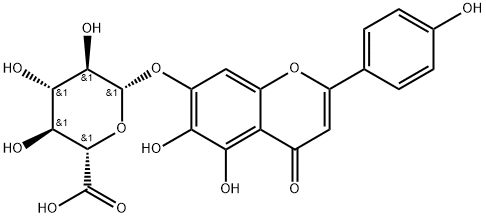
4',5,6,7-Tetrahydroxyflavone tetraacetate synthesis
- Product Name:4',5,6,7-Tetrahydroxyflavone tetraacetate
- CAS Number:1180-46-7
- Molecular formula:C23H18O10
- Molecular Weight:454.38
Yield:1180-46-7 90%
Reaction Conditions:
with pyridine;dmap at 0 - 25;Inert atmosphere;
Steps:
13 5,6,7-Triacetoxy-2-(4-acetoxyphenyl)-4H-chromen-4-one (19)
4.1.13
5,6,7-Triacetoxy-2-(4-acetoxyphenyl)-4H-chromen-4-one (19)
Pyridine (5 ml) and 4-dimethylaminopyridine (DMAP) (12.2 mg, 0.1 mmol) were added to a stirring solution of 2 (286 mg, 1.0 mmol) in acetic anhydride (5 ml) at 0 °C, then the mixture was warmed to room temperature.
After 4 h, the reaction mixture was poured into 100 ml water, and the solid obtained was filtered and purified by column chromatography (25% ethyl acetate in petroleum ether) to afford 19 (408 mg, 90% yield) as a yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 2.32 (s, 3H, -COCH3), 2.35 (s, 3H, -COCH3), 2.37 (s, 3H, -COCH3), 2.38 (s, 3H, -COCH3), 6.95 (s, 1H, 8-H), 7.36 (d, J = 8.7 Hz, 2H, 3',5'-H), 7.84 (s, 1H, 3-H), 8.14 (d, J = 8.7 Hz, 2H, 2',6'-H); ESI-MS: m/z 455 [M + H]+.
References:
Shi, Zhi-Hao;Li, Nian-Guang;Wang, Zhen-Jiang;Tang, Yu-Ping;Dong, Ze-Xi;Zhang, Wei;Zhang, Peng-Xuan;Gu, Ting;Wu, Wen-Yu;Yang, Jian-Ping;Duan, Jin-Ao [European Journal of Medicinal Chemistry,2015,vol. 106,p. 95 - 105]
27740-01-8
244 suppliers
$31.00/5mg

1180-46-7
9 suppliers
inquiry

642-71-7
199 suppliers
$6.00/100mg

1180-46-7
9 suppliers
inquiry