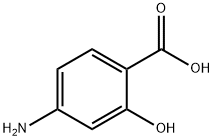
4-Aminosalicylic acid synthesis
- Product Name:4-Aminosalicylic acid
- CAS Number:65-49-6
- Molecular formula:C7H7NO3
- Molecular Weight:153.14
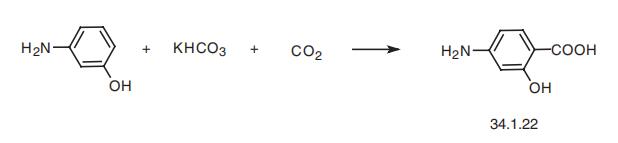
17980-39-1
20 suppliers
inquiry

65-49-6
471 suppliers
$6.00/25g
Yield:-
Reaction Conditions:
in toluene;
Steps:
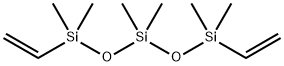
2 Example 2
Example 2
A reaction was performed in a manner similar to Example 1 except that a change was made to 25 g (0.0192 mol) of silsesquioxane derivative (DD-4H) manufactured in Synthesis Example 1, 51.3 g (0.197 mol) (10.3-fold moles based on the mole of DD-4 H) of DVTS, 25 g of toluene as a solvent, and further to a Pt concentration of 0.016 ppm based on DD-4H.
The reaction was tracked by GPC. After-treatment was carried out in a manner similar to Example 1 except that heating was stopped after 12 hours, and thus 33 g of colorless transparent liquid having a viscosity (at 25° C.) of 20 Pa·s was obtained.
Analysis of molecular weight by means of GPC yielded number average molecular weight:
Mn=1,381 and weight average molecular weight: Mw=1,660.
The colorless transparent liquid obtained is judged to have a structure of the formula (1-1) from analytical results as described below.
1H-NMR (solvent: CDCl3): δ (ppm); 0.0-0.6 (m, 60.7H), 5.0 (s, 2.0H), 5.8-6.4 (m, 4.6H), 7.05-7.50 (m, 40H).
References:
US2013/96249,2013,A1 Location in patent:Page/Page column
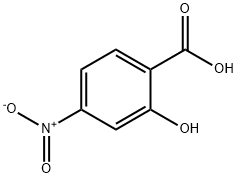
619-19-2
169 suppliers
$9.00/1g

65-49-6
471 suppliers
$6.00/25g
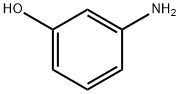
591-27-5
530 suppliers
$11.00/10g

65-49-6
471 suppliers
$6.00/25g

6018-19-5
224 suppliers
$9.00/25g

65-49-6
471 suppliers
$6.00/25g
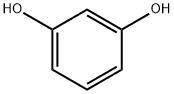
108-46-3
742 suppliers
$10.00/10g

65-49-6
471 suppliers
$6.00/25g