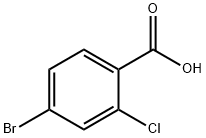
4-Bromo-2-chlorobenzoic acid synthesis
- Product Name:4-Bromo-2-chlorobenzoic acid
- CAS Number:59748-90-2
- Molecular formula:C7H4BrClO2
- Molecular Weight:235.46
201230-82-2
1 suppliers
inquiry

31928-47-9
204 suppliers
$10.00/5g

59748-90-2
273 suppliers
$8.00/1g
Yield:59748-90-2 76%
Reaction Conditions:
with water;palladium diacetate;triethylamine;triphenylphosphine in 1,4-dioxane at 110; under 11251.1 Torr; for 2 h;Flow reactor;
Steps:
Ortho-substituted carbonylation in flow using a “tube-in-tube” reactor
General procedure: For a typical reaction, a Vapourtec 2R+ Series was used as the platform with a Vapourtec Gas/Liquid Membrane Reactor to load the carbon monoxide. The HPLC pump were both set at 0.125 mL/min, temperature of the reactor at 110 °C, pressure of CO at 15 bar with a back pressure regulator of 250 psi (17.24 bar). The system was left running for 2 h to reach steady state after which time the flow streams were switched to pass from the loops where the substrates and catalysts were loaded. The first loop (5 mL) was filled with a solution of palladium acetate (20 mg, 0.08 mmol), triphenylphosphine (48 mg, 0.168 mmol) in 6 mL of 1,4-dioxane while the second loop (5 mL) was filled with a solution made from the ortho-substituted iodoarene substrate (1.68 mmol), triethylamine (0.272 g, 0.374 mL, 2.69 mmol) and water (0.505 g, 28 mmol) in 5.8 mL of 1,4-dioxane. An Omnifit column filled with 1.71 cm3 (r = 0.33 cm, h = 5.00 cm) of cotton was positioned just before the back pressure regulator to trap any particulate matter formed to avoid blocking of the back pressure regulator. After the substrates were passed through the system, the outlet of the flow stream was directed into a receptacle where the excess carbon monoxide gas was vented off in the fume cupboard. The reaction mixture was then evaporated to dryness, ethyl acetate (25 mL) and sodium carbonate solution (2 M, 10 mL) were added and transferred to a separating funnel. After collecting the aqueous layer, the organic layer was extracted with sodium carbonate solution (2 M, 2 × 10 mL). The combined aqueous layers were acidified by the addition of 2 M HCl solution which was then extracted with ethyl acetate (3 x 25 mL). The organic layer was dried over sodium sulfate, and the solvent evaporated under vacuum to give the crude product as a solid. The crude product was then recrystallised from the appropriate solvent.
References:
Mallia, Carl J.;Walter, Gary C.;Baxendale, Ian R. [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 1503 - 1511] Location in patent:supporting information

89794-02-5
230 suppliers
$6.00/1g

59748-90-2
273 suppliers
$8.00/1g

119-32-4
253 suppliers
$15.00/25g

59748-90-2
273 suppliers
$8.00/1g