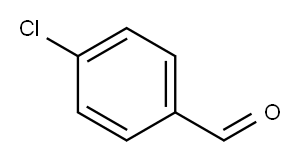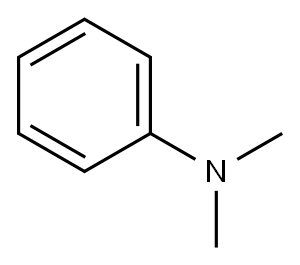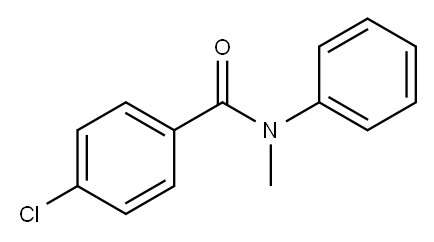
4-chloro-N-methyl-N-phenylbenzamide synthesis
- Product Name:4-chloro-N-methyl-N-phenylbenzamide
- CAS Number:1517-46-0
- Molecular formula:C14H12ClNO
- Molecular Weight:245.7
Yield:1517-46-0 87%
Reaction Conditions:
with tert.-butylhydroperoxide;tetra-(n-butyl)ammonium iodide in water;ethyl acetate at 90; for 24 h;
Steps:
General procedure for amides:
General procedure: amine (0.5 mmol) and aldehyde (2.5 mmol) were added to a tube with a mixture of nBu4NI (4.6 mg, 0.0125 mmol) and EtOAc (3.0 mL) at roomtemperature. Then tert-butyl hydroperoxide (TBHP in water, 70 wt%) was droppedinto the mixture. The resulting mixture was stirred at 90 °C for 24h. The temperature of the reaction was cooled to room temperature. Theresulting reaction solution was concentrated in vacuo. The resulting residuewas purified by flash column chromatography on silica gel with ethylacetate/petroleum ether (1:10) as an eluent to afford the amide product.
References:
Wang, Shan;Wang, Jian;Guo, Rui;Wang, Gao;Chen, Shan-Yong;Yu, Xiao-Qi [Tetrahedron Letters,2013,vol. 54,# 46,p. 6233 - 6236] Location in patent:supporting information