4-METHOXY-N-2-NAPHTHALENYL-BENZAMIDE synthesis
- Product Name:4-METHOXY-N-2-NAPHTHALENYL-BENZAMIDE
- CAS Number:108717-14-2
- Molecular formula:C18H15NO2
- Molecular Weight:277.32
Yield:108717-14-2 60%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane;
Steps:
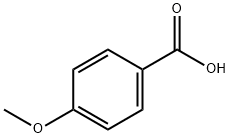
4-Methoxy-N-(naphthalen-2-yl)benzamide (24).
To a stirred solution of 4-methoxybenzoic acid (100mg, 0.66 mmol) in DCM (5mL) was added EDCI (127 mg, 0.66 mmol), HOBt (27 mg, 0.20 mmol), TEA(91 μL, 0.66 mmol), and naphthalen-2-amine (89 μL, 0.66 mmol). The reaction mixture was stirredovernight and then diluted with DCM (5 mL). The reaction mixture was washed with H2O (5 mL) andbrine (5 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reducedpressure. The resulting residue was purified by flash chromatogray using 2:1 EtOAc/Hex to afford a solid(110 mg, 60% yield). 1H NMR (400 MHz, DMSO) δ 10.30 (s, 1H), 8.45 (d, J = 5 Hz, 1H), 8.04 (d, J = 22Hz, 2H), 7.88 - 7.84 (m, 4H), 7.48 - 7.41 (m, 2H), 7.10 (d, J = 22 Hz, 2H), 3.83 (s, 3H). 13C NMR (100MHz, DMSO) δ 165.1, 162.0, 137.0, 133.3, 129.8, 129.6, 128.0, 127.4, 127.3, 126.9, 126.3, 124.6, 121.0,116.4, 113.6, 55.4. HRMS Calcd for C18H15NO2 (MH+): 278.3251, Found: 278.1183.
References:
Naro, Yuta;Thomas, Meryl;Stephens, Matthew D.;Connelly, Colleen M.;Deiters, Alexander [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 21,p. 4793 - 4796] Location in patent:supporting information
100-07-2
389 suppliers
$35.00/25g
91-59-8
194 suppliers
$61.39/100mg

108717-14-2
3 suppliers
inquiry