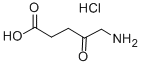
5-Aminolevulinic acid hydrochloride synthesis
- Product Name:5-Aminolevulinic acid hydrochloride
- CAS Number:5451-09-2
- Molecular formula:C5H10ClNO3
- Molecular Weight:167.59

Shrestha-dawadi, Prativa Bade; Lugtenburg, Johan. Preparation of [1,2,3,4,5-13C5]-5-amino-4-oxopentanoic acid (ALA) - design of a synthetic scheme to prepare any 13C- and 15N-isotopomer with high isotopic enrichment. European Journal of Organic Chemistry. Issue 23. Pages 4654-4663. 2003.

92632-81-0
26 suppliers
inquiry

5451-09-2
488 suppliers
$16.80/1G
Yield:5451-09-2 96.6%
Reaction Conditions:
with hydrogenchloride in water; for 6 h;Reflux;
Steps:
2.1; 2.1-1; 2.2; 2.1-2; 2.3-2.17; 2.1-3; 2.5.3; 3
Dissolve 2.00 g of 5-phthalimide levulinic acid obtained in the previous step with 40 ml of 6N hydrochloric acid and heat it for reaction at reflux for 6 hours, decolorize and filter on activated carbon, distill under reduced pressure to remove water to the end, and recrystallize the remainder with acetone , To obtain 1.24 g of solid. According to the proton nuclear magnetic resonance spectrum data, the obtained solid is determined to be 5-aminolevulinic acid hydrochloride, with a yield of 96.6% (calculated as 5-phthalimidolevulinic acid); mp: 149°C, purity ( HPLC, a/a%) 99.9%.
References:
CN111592484,2020,A Location in patent:Paragraph 0060; 0062-0063; 0065-0066; 0068-0069; 0071-0072

102872-00-4
9 suppliers
inquiry

5451-09-2
488 suppliers
$16.80/1G

20238-92-0
0 suppliers
inquiry

5451-09-2
488 suppliers
$16.80/1G

839724-53-7
0 suppliers
inquiry

5451-09-2
488 suppliers
$16.80/1G

109258-71-1
33 suppliers
$263.00/500mg

5451-09-2
488 suppliers
$16.80/1G








