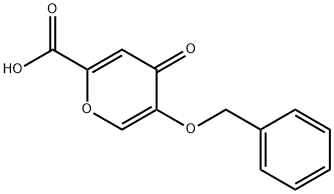
5-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid synthesis
- Product Name:5-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid
- CAS Number:1219-33-6
- Molecular formula:C13H10O5
- Molecular Weight:246.22
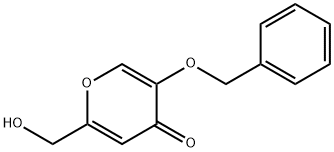
15771-06-9
56 suppliers
$25.00/250mg
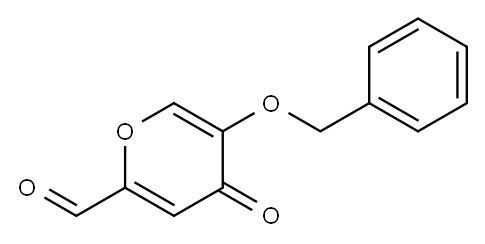
18234-41-8
8 suppliers
inquiry

1219-33-6
57 suppliers
$72.00/100mg
Yield: 38% , 6 %Chromat.
Reaction Conditions:
with potassium bromate;sodium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;tetrabutylammomium bromide;sodium hydrogencarbonate in dichloromethane;water at 0; for 0.1 - 0.15 h;Product distribution / selectivity;
Steps:
2.1; A.a
EXAMPLE 2 Preparation of 5-benzyloxy-4-oxo-4H-pyran-2-carbaldehyde Method 1-TEMPO oxidation: Solid NaHCO3 (96.0 g, 1.21 mol) and deionized water (125 mL) were added to a mixture of 5-benzyloxy-2-hydroxymethyl-pyran-4-one (40.0 g, 0.17 mol) in CH2Cl2 (550 mL) and deionized water (200 mL). The mixture was cooled in an ice-salt bath to about 0° C. Then, KBrO3 (4.00 g, 24.1 mmol), n-Bu4N+Br- (2.20 g, 6.90 mmol) and TEMPO (0.54 g, 3.44 mmol) were successively added. To the resulting heterogeneous mixture at 0° C. was added a solution of 1.59 M NaOCl (140 mL, 1.3 equiv) within 5-7 min. The reaction mixture turned to orange then to bright yellow during the NaOCl addition. The mixture was stirred for an additional 1-2 min, and then the solid was filtered off by suction filtration. The filtrate was placed in a separatory funnel, and the organic fraction was collected and dried over sodium sulfate. Analysis of the crude mixture by HPLC Method 1 (λ=280 nm), as described above, indicated the presence of the over-oxidized product (5-benzyloxy-4-oxo-4H-pyran-2-carboxylic acid, peak percent area: 6%, RT=9.42 min), the desired product (5-benzyloxy-4-oxo-4H-pyran-2-carbaldehyde, peak percent area is 85%, and its RT is 11.34 min) and the starting material (5-benzyloxy-2-hydroxymethyl-pyran-4-one, peak percent area is 8%, and its RT is 12.18 min). The organic fraction was filtered and the volume was reduced to about 60 mL using the rotary evaporator. The mixture was placed on top of a wet-packed silica gel column, and eluted with ethyl acetate. The fractions containing the product were combined. Upon concentration on the rotary evaporator (volume reduction), the product separated out as a solid. The solid was collected by suction filtration to provide a first crop (10 g after drying under vacuum). The filtrate was collected and the volume of solvent reduced under vacuum. The precipitated solid was collected by filtration to give a second crop (5 g after drying under vacuum). The crops were combined to provide a 38% yield of the titled compound. The purity (peak area percent is 97.4%) of this material was analyzed by HPLC Method 1 as described above. 1H NMR (400 MHz, DMSO-D6) δ (ppm): 9.64 (s, 1H), 8.41 (s, 1H), 7.32-7.43 (m, 5H), 7.13 (s, 1H), 5.01 (s, 2H, CH2Ph).
References:
Apotex Technologies Inc. US2008/242706, 2008, A1 Location in patent:Page/Page column 12; 19; 39

15771-06-9
56 suppliers
$25.00/250mg

1219-33-6
57 suppliers
$72.00/100mg

501-30-4
609 suppliers
$5.00/25mg
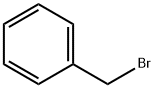
100-39-0
424 suppliers
$10.00/10 g

1219-33-6
57 suppliers
$72.00/100mg

501-30-4
609 suppliers
$5.00/25mg

1219-33-6
57 suppliers
$72.00/100mg
100-44-7
626 suppliers
$13.50/250G

1219-33-6
57 suppliers
$72.00/100mg