
5-Butyl-3-phenylisoxazole synthesis
- Product Name:5-Butyl-3-phenylisoxazole
- CAS Number:1017-10-3
- Molecular formula:C13H15NO
- Molecular Weight:201.26
Yield:1017-10-3 83%
Reaction Conditions:
Stage #1: Benzaldoximewith N-chloro-succinimide;TPGS-750-M in water at 20; for 4 h;
Stage #2: hex-1-ynewith triethylamine in water at 20; for 8 h;
Steps:
32 Example 32: Synthesis of 5-butyl-3-phenylisoxazole
Add 24.1mg (0.18mmol) NCS and 18.2mg (0.15mmol) benzaldehyde oxime (I-1) to a 5mL reaction flask, and then add 1mL of newly prepared 2wt.% TPGS-750-M aqueous solution into the flask, And the mixture was stirred at room temperature for 4 hours. Four hours later, 14 μL (0.1 mmol) Et3N, 8.2 mg (0.1 mmol) 1-hexyne (II-11) were added in sequence, and the reaction was carried out at room temperature for 8 hours. After the reaction is over, add 2mL ethyl acetate to the reaction flask, stir for 5min and then stand for separation. After collecting the upper organic phase, add 2mL ethyl acetate to the lower aqueous phase, stir for 5min and then stand for separation and collect the upper organic phase. After that, the organic phases obtained twice were combined, the solvent was evaporated under reduced pressure, and the solid residue was separated by column chromatography. The eluent was petroleum ether: ethyl acetate = 1:2. The eluent containing the target product was collected and reduced. The solvent was evaporated under pressure to obtain 16.7 mg of 5-butyl-3-phenylisoxazole represented by formula (III-21) as a colorless oily substance with a yield of 83%. HPLC purity is 96.2%
References:
CN113582937,2021,A Location in patent:Paragraph 0145-0148
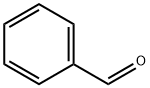
100-52-7
947 suppliers
$20.37/250g

1017-10-3
1 suppliers
inquiry

18998-78-2
0 suppliers
inquiry

1017-10-3
1 suppliers
inquiry

873-67-6
4 suppliers
inquiry

1191-12-4
0 suppliers
inquiry

1017-10-3
1 suppliers
inquiry

2039-49-8
5 suppliers
$172.00/25MG

