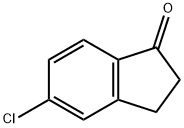
5-Chloro-1-indanone synthesis
- Product Name:5-Chloro-1-indanone
- CAS Number:42348-86-7
- Molecular formula:C9H7ClO
- Molecular Weight:166.6
Synthetic method of 5-chloro-1-indanone

7448-87-5
2 suppliers
inquiry

42348-86-7
376 suppliers
$9.00/1g
Yield:42348-86-7 95.1%
Reaction Conditions:
with hydrogenchloride in toluene at -5 - 10; under 1500.15 - 1875.19 Torr; for 4 h;Temperature;Solvent;
Steps:
1-4 Example 2
(1) Weigh 175.2 g (1.0 mol) of 1-(4-chlorophenyl)-2-propene-1-one with a purity of 95% and 400 g of toluene, place them in a high-pressure reaction flask, and stir to dissolve them. (2) Put the reaction flask in a low-temperature tank and stir and cool down. When the temperature in the reaction flask drops to -50, start to pass hydrogen chloride gas slowly, set the aeration rate to 0.1L/min, and increase the system temperature and keep it at 510 Through hydrogen chloride reaction. (3) The temperature of the system no longer increased after 2 hours of aeration reaction. A sample was taken to detect the residual 15.4% of 1-(4-chlorophenyl)-2-propene-1-one. Close the tail gas vent valve of the reaction flask and continue to pass hydrogen chloride gas. When the pressure of the high-pressure reaction flask reaches 0.2-0.25MPa, close the hydrogen chloride vent valve, and continue the heat preservation reaction at 0-5°C for 2 hours. (4) Take a sample to detect the residual 0.3% of 1-(4-chlorophenyl)-2-propene-1-one, and the reaction ends. The pH of the system was adjusted to 7-8 with 5% sodium hydroxide solution and stirred for 0.5 h, filtered and dried to obtain 160.9 g of white solid 5-chloro-1-indanone, with a purity of 98.4% by HPLC and a yield of 95.1%.
References:
CN113087609,2021,A Location in patent:Paragraph 0025-0044
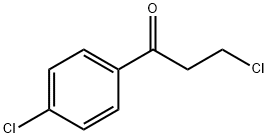
3946-29-0
128 suppliers
$10.00/1g

42348-86-7
376 suppliers
$9.00/1g
40478-50-0
3 suppliers
inquiry

42348-86-7
376 suppliers
$9.00/1g

21640-48-2
144 suppliers
$5.00/250mg

42348-86-7
376 suppliers
$9.00/1g

108-90-7
642 suppliers
$10.00/25G
625-36-5
358 suppliers
$45.00/5g

42348-86-7
376 suppliers
$9.00/1g