6-BROMOFLAVONE synthesis
- Product Name:6-BROMOFLAVONE
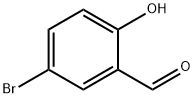
- CAS Number:1218-80-0
- Molecular formula:C15H9BrO2
- Molecular Weight:301.13
Yield:1218-80-0 92%
Reaction Conditions:
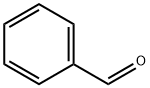
Stage #1: 2-(dimethyl(oxo)-λ6-sulfaneylidene)-1-phenylethan-1-one;5-bromosalicyclaldehydewith dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer;cesium acetate in 1,2-dichloro-ethane at 100; for 6 h;Sealed tube;Inert atmosphere;
Stage #2: with sulfuric acid;acetic acid in 1,2-dichloro-ethane at 100; for 3 h;Sealed tube;Inert atmosphere;
Steps:
General procedure for the synthesis of 3
General procedure: A sealed tube was charged with 1a (0.2 mmol, 1.0 equiv.), 2a (0.3 mmol, 1.5 equiv.), [Cp*RhCl2]2 (0.005 mmol, 2.5 mol%), and CsOAc (0.1 mmol, 0.5 equiv.) in DCE (2 mL). After the reaction mixture had been stirred at 100 °C under Ar for 6 h, 1 mL of H2SO4/HOAc (10 μL of H2SO4 dissolved in 0.99 mL of HOAc) was added at room temperature. After completion of the addition, the reaction mixture was then stirred at 100 °C for 3 h before being cooled to room temperature. The mixture was diluted with EtOAc (20 mL), washed with brine, and dried over anhydrous Na2SO4. After removal of the EtOAc, the residue was purified by chromatography on basic silica gel (PE/EtOAc = 8/1) to afford 3aa (40 mg, 89%) as a white solid.
References:
Cheng, Kang;Chen, Jinkang;Jin, Licheng;Zhou, Jian;Jiang, Xinpeng;Yu, Chuanming [Journal of Chemical Research,2019,vol. 43,# 9-10,p. 392 - 398]

5067-24-3
24 suppliers
inquiry

1218-80-0
34 suppliers
$60.00/2g

109619-11-6
0 suppliers
inquiry

1218-80-0
34 suppliers
$60.00/2g