
7-OCTENOIC ACID synthesis
- Product Name:7-OCTENOIC ACID
- CAS Number:18719-24-9
- Molecular formula:C8H14O2
- Molecular Weight:142.2

821-41-0
269 suppliers
$15.00/1g
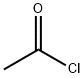
75-36-5
560 suppliers
$17.92/100G

18719-24-9
64 suppliers
$40.00/100mg
Yield: 99%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 18 h;
Steps:
F3.i
F3) Synthesis of 5,6-Diazido-hexan-1-ol(i) Hex-5-enyl-1-acetateTo an anhydrous solution of hex-5-en-1-ol (15.0 g, 0.15 mol) in dichloromethane (250 ml) was added acetyl chloride (11.3 ml, 0.20 mol), Triethylamine (20.8 ml, 0.15 ml) was then added and the reaction mixture was stirred at room temperature (18 h) followed by the addition of water (50 ml). The organic layer was separated, the aqueous layer extracted with dichloromethane (2×50 ml) and the combined organic extracts were washed with saturated brine solution (3×100 ml). The organic layer was finally washed with water (3×100 ml) and dried over anhydrous magnesium sulphate. On removal of the drying agent by filtration under gravity, the solvent was removed in vacuo to afford hex-5-enyl-1-acetate as a colourless oil, in near quantitative yield (21.0 g, 99%).δH (CDCl3): 1.32 (2H, m) [H3]; 1.53 (2H, m) [H4]; 1.94-1.97 (5H, m) [CH3CO, H2], 3.94 (2H, t, J 6.4 Hz) [H1]; 4.88 (2H, m) [H6]; 5.68 (1H, m) [H5].δC (CDCl3): 20.89 [CH3CO]; 25.17 [C3]; 28.02 [C4]; 33.28 [C2]; 64.31 [C1]; 114.78 [C6]; 138.25 [C5]; 171.01 [CO].
References:
The Secretary of State for Defense US2012/53356, 2012, A1 Location in patent:Page/Page column 11
![Bicyclo[5.1.0]octane, 1-[(trimethylsilyl)oxy]-](/CAS/20200611/GIF/50338-48-2.gif)
50338-48-2
0 suppliers
inquiry

18719-24-9
64 suppliers
$40.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

18719-24-9
64 suppliers
$40.00/100mg
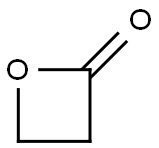
57-57-8
159 suppliers
$24.73/250mgs:
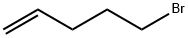
1119-51-3
353 suppliers
$12.00/5g

18719-24-9
64 suppliers
$40.00/100mg
![Bicyclo[5.1.0]octan-1-ol](/CAS/20210305/GIF/50338-54-0.gif)
50338-54-0
0 suppliers
inquiry

18719-24-9
64 suppliers
$40.00/100mg