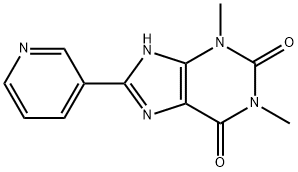
8-(3-Pyridyl)theophyline synthesis
- Product Name:8-(3-Pyridyl)theophyline
- CAS Number:1029-62-5
- Molecular formula:C12H11N5O2
- Molecular Weight:257.25
Yield:1029-62-5 78%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in water;acetonitrile at 30 - 35; for 2 h;
Steps:
4.2. Typical synthetic procedure for 8-substituted xanthines
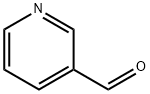
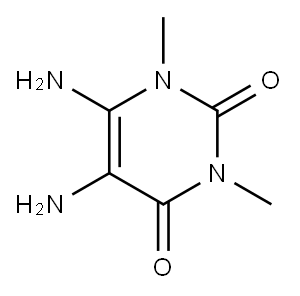
General procedure: In a round bottom flask, 5,6-diamino-1,3-dimethyluracil (0.425 g, 2.5 mmol, 1 equiv) and aryl/cycloaryl/heteroaryl aldehyde (2.5 mmol, 1 equiv) was stirred in CH3CN/H2O (9:1) (10 mL) for 15 min. To this, catalytic amount (0.008 g, 0.05 mmol, 0.02 equiv) of AIBN was added and after stirring for 5 min, 0.7 equiv of NBS (in two portions, initially 0.5 equiv and after 30 min additional 0.2 equiv) (0.311 g, 1.75 mmol) was added and the reaction mixture was stirred at room temperature (30-35 °C). The reaction progress was monitored by TLC (dichloromethane/methanol=19:1) as well as HPLC. After the reaction was completed, the resulting precipitate was filtered under vacuum and the precipitate was washed with ethyl acetate (2×10 mL) and methanol (3×10 mL) to obtain the pure product.
References:
Bandyopadhyay, Prabal;Agrawal, Sumit K.;Sathe, Manisha;Sharma, Pratibha;Kaushik [Tetrahedron,2012,vol. 68,# 20,p. 3822 - 3827] Location in patent:supporting information; experimental part