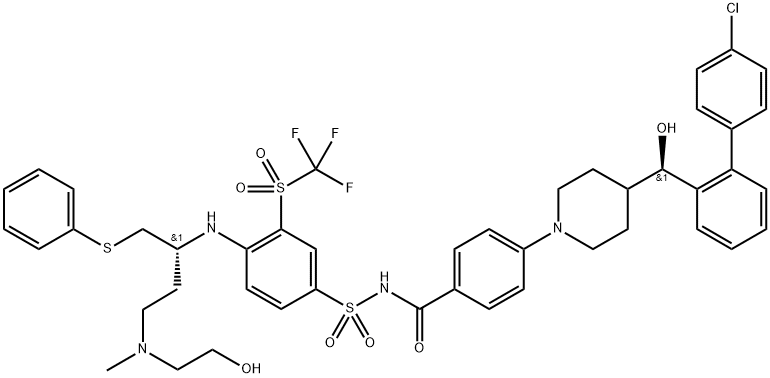
AZD4320 synthesis
- Product Name:AZD4320
- CAS Number:1357576-48-7
- Molecular formula:C45H48ClF3N4O7S3
- Molecular Weight:945.53
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1357576-48-7
22 suppliers
inquiry
Yield: 80%
Reaction Conditions:
Stage #1:N-(4-((R)-4-((2-(tert-butyldimethylsilyloxy)ethyl)(methyl)amino)-1-(phenylthio)butan-2-ylamino)-3-(trifluoromethylsulfonyl)phenylsulfonyl)-4-(4-((R)-(4'-chlorobiphenyl-2-yl)(hydroxy)methyl)piperidin-1-yl)benzamide with hydrogenchloride in 1,4-dioxane;methanol at 0 - 25;
Stage #2: with sodium hydrogencarbonate in 1,4-dioxane;methanol;water;ethyl acetate
Steps:
3 4-(4-((R)-(4'-chlorobiphenyl-2-yl)(hydroxy)methyl)piperidin-1-yl)-N-(4-((R)-4-((2-hydroxyethyl)(methyl)amino)-1-(phenylthio)butan-2-ylamino)-3-(trifluoromethylsulfonyl)phenylsulfonyl)benzamide
To a solution of N-(4-((R)-4-((2-(tert-butyldimethylsilyloxy)ethyl)(methyl)amino)-1-(phenylthio)butan-2-ylamino)-3-(trifluoromethylsulfonyl)phenylsulfonyl)-4-(4-((R)-(4'-chlorobiphenyl-2-yl)(hydroxy)methyl)piperidin-1-yl)benzamide (INTERMEDIATE 34, 2.96 g, 2.51 mmol) in dioxane (5.03 ml) and methanol (5.03 ml) was added slowly HCl (4M in dioxane, 15.08 ml, 60.33 mmol). Approximately half way through addition, a slight exotherm was noted, and the reaction was cooled to 0° C. Addition of hydrochloric acid in dioxane (4M) continued, and once complete, the ice bath was removed. The resulting yellow solution was allowed to warm to r.t. and stirred for 3 min before being concentrated under reduced pressure to half volume and diluted with ethyl acetate. The resulting mixture was washed with sodium bicarbonate (saturated, aqueous) and sodium chloride (saturated, aqueous) before being dried over sodium sulfate, filtered, and concentrated. Upon concentration, a thick oily residue precipitated out of solution and this material was redissolved in acetone before being reconcentrated to a beige film. This film was dissolved in 10% methanol in ethyl acetate and purified by column chromatography (ISCO, 330 g SiO2, isocratic 15% methanol in ethyl acetate for 30 min) to afford the title compound (1.90 g, yield: 80%). 1H NMR (300 MHz, METHANOL-d4) δ 8.27 (s, 1H), 8.04 (dd, 1H), 7.82 (d, 2H), 7.62 (d, 1H), 7.09-7.50 (m, 12H), 6.66-6.86 (m, 3H), 4.44 (d, 1H), 3.99 (br. s., 1H), 3.69-3.85 (m, 3H), 3.62 (d, 1H), 3.12-3.29 (m, 2H), 2.86-3.05 (m, 4H), 2.60-2.75 (m, 4H), 2.46-2.58 (m, 1H), 2.11-2.28 (m, 1H), 1.88-2.12 (m, 2H), 1.65-1.84 (m, 1H), 1.08-1.35 (m, 2H), 0.88-1.07 (m, 1H). 19F NMR (282 MHz, METHANOL-d4) δ -80.88 (3F). LCMS: (ESI) m/z 945.4, 947.4 [M+H]+.
References:
ASTRAZENECA AB US2012/35134, 2012, A1 Location in patent:Page/Page column 79; 80
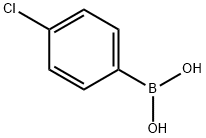
1679-18-1
588 suppliers
$10.00/1g

1357576-48-7
22 suppliers
inquiry
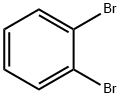
583-53-9
309 suppliers
$10.00/5g

1357576-48-7
22 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1357576-48-7
22 suppliers
inquiry
![Benzenesulfonamide, 4-[[(1R)-3-oxo-1-[(phenylthio)methyl]propyl]amino]-3-[(trifluoromethyl)sulfonyl]-](/CAS/20210305/GIF/1357575-44-0.gif)
1357575-44-0
0 suppliers
inquiry

1357576-48-7
22 suppliers
inquiry