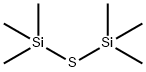
BIS(TRIMETHYLSILYL) SULFIDE synthesis
- Product Name:BIS(TRIMETHYLSILYL) SULFIDE
- CAS Number:3385-94-2
- Molecular formula:C6H18SSi2
- Molecular Weight:178.44
Yield:3385-94-2 70%
Reaction Conditions:
with lithium;sulfur in tetrahydrofuran at 0 - 50;Inert atmosphere;Glovebox;Temperature;
Steps:
1.1; 1.2; 1.3; 1.4; 2.1; 2.2; 2.3; 2.4; 3.1; 3.2; 3.3; 3.4; 4.1; 4.2; 4.3; 4.4; 5.1; 5.2; 5.3; 5.4; 6
Under the protection of inert gas, put 16g sublimed sulfur powder (0.5mol) and 100mL tetrahydrofuran into a 500mL four-neck flask, and cool the internal temperature of the system to 0°C with an ice salt bath. Under the protection of strict inert gas, weigh 6.94g of active lithium powder (4-16 mesh) into the above-mentioned 500mL reaction flask in a glove box. Put 127 mL of trimethylchlorosilane (1.0 mol) into a constant pressure dropping funnel and connect with a 500 mL four-necked flask. The internal temperature was controlled to 1°C, and 5 drops of trimethylchlorosilane (about 0.14 mL) were added dropwise. At this time, the reaction system turned dark orange, but the temperature increase of the system was not obvious. After reacting for 5 minutes, continue to add 6 drops of trimethylchlorosilane, the color of the system gradually deepens, but the temperature still does not rise significantly. After the reaction continued for 5 minutes, 11 drops of trimethylchlorosilane were added dropwise. At this time, the system exothermed a lot. The temperature of the system increased rapidly by 17°C within 5 minutes, the color continued to deepen to reddish brown, and the sublimated sulfur powder gradually dissolved. After the sulfur powder is completely dissolved, the temperature of the reaction system decreases. So far, 0.6 mL of trimethylchlorosilane was added dropwise cumulatively. After the system temperature stabilizes, start to slowly add the remaining trimethylchlorosilane dropwise to maintain the system temperature at about 15°C. After dropping to about 40 mL, remove the cooling bath. The remaining about 85mL is added dropwise when the temperature of the control system is lower than 50, and the dropping speed is as slow as possible. After dropping to about 2/3 of trimethylchlorosilane, the temperature of the system drops slightly. At this time, it is necessary to stop the dropping to allow the reaction to continue for a period of time, and use the heat of reaction to increase the temperature. When the last 10% trimethylchlorosilane is left, it needs to be dripped at a rate of 1-2 drops/min. It takes 11 hours to add 127 mL of trimethylchlorosilane dropwise. After the addition was completed, the system continued to react overnight. After the reaction is completed, the solvent tetrahydrofuran is first distilled under normal pressure, and then distilled under reduced pressure to obtain the hexamethyldisilicate product. The yield was 70%, and the purity of the product was 99.4% by GC analysis. The trace impurities were trimethylchlorosilane and hexamethyldisiloxane.
References:
CN112940028,2021,A Location in patent:Paragraph 0005; 0026-0064
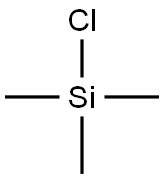
75-77-4
592 suppliers
$5.04/10

3385-94-2
149 suppliers
$43.00/1g
16029-98-4
404 suppliers
$29.00/5g

3385-94-2
149 suppliers
$43.00/1g

188290-36-0
2 suppliers
inquiry

75-77-4
592 suppliers
$5.04/10

3385-94-2
149 suppliers
$43.00/1g

114142-37-9
0 suppliers
inquiry