
BOC-4-AMINOBENZYLALCOHOL synthesis
- Product Name:BOC-4-AMINOBENZYLALCOHOL
- CAS Number:144072-29-7
- Molecular formula:C12H17NO3
- Molecular Weight:223.27

24424-99-5
816 suppliers
$13.50/25G
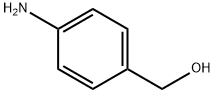
623-04-1
347 suppliers
$6.00/1g

144072-29-7
85 suppliers
$8.00/250mg
Yield: 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuranHeating / reflux;
Steps:
10
To a solution of p-amino-benzylalcohol (1 g, 8.12 mmol, 1 eq) in 80 mL of anhydrous THF were added DIEA (1.4 mL, 8.12 mmol, 1 eq) and BoC2O (1.9 mL, 8.12 mmol, 1 eq). The mixture was heated to reflux overnight, then cooled down and evaporated under vacuum. The residue was dissolved in EtOAc. The organic layer was washed with a 0.1 N HCl solution, dried over MgSO4, filtered and evaporated under vacuum. The crude product was purified by
References:
ENZON PHARMACEUTICALS, INC. WO2008/34124, 2008, A2 Location in patent:Page/Page column 72-73

144072-30-0
94 suppliers
$22.00/250mg

144072-29-7
85 suppliers
$8.00/250mg
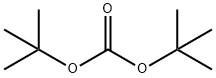
34619-03-9
26 suppliers
inquiry

623-04-1
347 suppliers
$6.00/1g

144072-29-7
85 suppliers
$8.00/250mg

110969-44-3
17 suppliers
$333.00/5g

144072-29-7
85 suppliers
$8.00/250mg

24424-99-5
816 suppliers
$13.50/25G
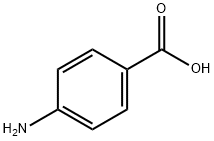
150-13-0
880 suppliers
$5.00/25g

144072-29-7
85 suppliers
$8.00/250mg