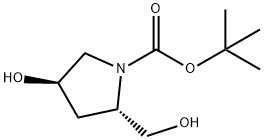
BOC-HYP-OL synthesis
- Product Name:BOC-HYP-OL
- CAS Number:61478-26-0
- Molecular formula:C10H19NO4
- Molecular Weight:217.26

74844-91-0
402 suppliers
$5.00/5g

61478-26-0
215 suppliers
$6.00/250mg
Yield:61478-26-0 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at -16; for 3.5 h;Inert atmosphere;
Steps:
19 Intermediate 19tert-Butyl (2S,4R)-4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate
To a stirred solution of 1 -tert-butyl 2-methyl (2S,4R)-4-hydroxypyrrolidine-1 2- dicarboxylate (5.0 g, 20.41 mmol) in THF (100 mL) under an argon atmosphere at -16°C was added LiAIH4 (1 M in THF, 21 mL, 21 mmol) dropwise over 5 mm.A precipitate was formed that was broken up by the addition of THF (50 mL). The reaction mixture was stirred for 3.5 h, during which time the temperature was allowed to warm to 0°C. The reaction was quenched by the addition of EtOAc (5 mL), and stirred for 30 mm. Excess Rochelles salt solution (100 mL) was carefully added and the reaction mixture allowed to warm to rt and stirredfor a further 1 h. The product was isolate by extraction twice with CH2CI2 (100 mL) and the combined organic fractions dried over Mg504, filtered and the solvent removed by evaporation in vacuo. Purification by column chromatography, 10% MeOH/CH2CI2, gave Intermediate 19 in quantitative yield. 1H NMR (300MHz, DMSO-d5) 0H. 4.82 (br 5, 1 H), 4.64 (br d, J=5.5 Hz, 1 H),4.20 (sxt, J=4.2 Hz, 1 H), 3.75 (brs, 1 H), 3.16-3.50 (m, 4 H), 1.85-2.02 (m, 1 H),1 .80 (br 5, 1 H), 1 .39 (5, 9 H).
References:
WO2017/29521,2017,A1 Location in patent:Page/Page column 37
13726-69-7
429 suppliers
$5.00/1g

61478-26-0
215 suppliers
$6.00/250mg

24424-99-5
815 suppliers
$13.50/25G
51-35-4
820 suppliers
$6.00/5g

61478-26-0
215 suppliers
$6.00/250mg

37813-30-2
87 suppliers
$28.00/1g

61478-26-0
215 suppliers
$6.00/250mg

186320-37-6
0 suppliers
inquiry

61478-26-0
215 suppliers
$6.00/250mg