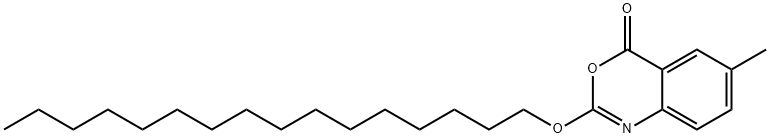
Cetilistat synthesis
- Product Name:Cetilistat
- CAS Number:282526-98-1
- Molecular formula:C25H39NO3
- Molecular Weight:401.58
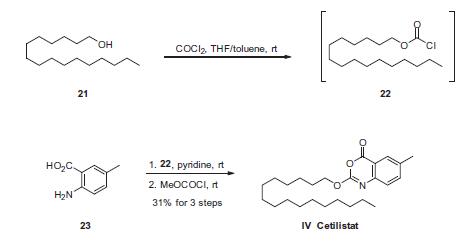

890655-08-0
11 suppliers
inquiry

282526-98-1
425 suppliers
$5.00/250mg
Yield:282526-98-1 96.5%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20;Solvent;Reagent/catalyst;
Steps:
5 Example 5 Synthesis of Cetilistat
In a 3L three-mouth flask, disperse the compound C (120g) in 2L dichloromethane. Under stirring add EDCI (82.24g). The reaction was stirred at room temperature.TLC (n-hexane: ethyl acetate: acetic acid = 50:1:0.1) monitored the progress of the reaction, the reaction was complete after washing 3 times × 700mL, the organic layer was separated, 12g of activated carbon was added and refluxed for 20min, cooled to room temperature activated carbon was filtered off , concentrated under reduced pressure to remove methylene chloride, the crude product was dispersed in 575mL of acetonitrile, stirred at room temperature for 1 hour to fully disperse the substrate, an ice-water bath (0-10 deg.C) was stirred for 3 hours, filtration, 40 deg.C blast drying, a white solid 110.8g. Yield 96.5%.
References:
CN105669585,2016,A Location in patent:Paragraph 0063; 0064; 0065

890655-08-0
11 suppliers
inquiry
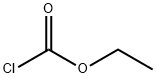
541-41-3
6 suppliers
$21.00/5g

282526-98-1
425 suppliers
$5.00/250mg
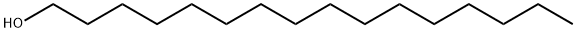
36653-82-4
499 suppliers
$6.00/100g

282526-98-1
425 suppliers
$5.00/250mg

26272-90-2
129 suppliers
$71.00/1g

282526-98-1
425 suppliers
$5.00/250mg

13784-52-6
0 suppliers
inquiry

282526-98-1
425 suppliers
$5.00/250mg