DBU synthesis
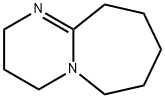
- Product Name:DBU
- CAS Number:6674-22-2
- Molecular formula:C9H16N2
- Molecular Weight:152.24


24566-95-8
55 suppliers
$68.00/100mg

6674-22-2
587 suppliers
$5.00/10g
Yield:6674-22-2 91.7 %
Reaction Conditions:
with toluene-4-sulfonic acid in water at 150 - 160;Dean-Stark;chemoselective reaction;Reagent/catalyst;Temperature;
Steps:
3; 6-7 Example 3: Dehydration of crude amine solution in xylene
To 159.4 g of solution obtained as described in example 2, 1.3 g of p- toluenesulphonic acid monohydrate was added. This mixture was heated to 150- 160°C by reflux through the use of a dry-half spherical heater connected to a temperature controller; the reaction flask was connected to a Dean-Stark trap and a condenser to remove the water produced from the reaction environment: the vapours produced condensed on top of the trap and the water thus formed was removed by gravity into the trap (the solvent fell back into the flask maintaining an almost constant volume). After 4 h from the start of reflux, no further accumulation of water was observed in the trap and the reaction was considered to be complete, then allowed to cool to room temperature. The resulting solution was neutralised with 800 mg of aqueous NaOH solution (min. concentration 45%). GC-MS analysis calculated a conversion of N-(3-aminopropyl)-ε- caprolactam of 92.3%, a selectivity of 99.4% and thus a DBU yield of 91.7%.
References:
WO2022/189911,2022,A1 Location in patent:Page/Page column 24-26; 29

105-60-2
509 suppliers
$5.00/25g

107-13-1
393 suppliers
$17.00/25ML

24566-95-8
55 suppliers
$68.00/100mg

6674-22-2
587 suppliers
$5.00/10g

105-60-2
509 suppliers
$5.00/25g

107-13-1
393 suppliers
$17.00/25ML

6674-22-2
587 suppliers
$5.00/10g

7336-15-4
33 suppliers
inquiry

24566-95-8
55 suppliers
$68.00/100mg

6674-22-2
587 suppliers
$5.00/10g

105-60-2
509 suppliers
$5.00/25g

6674-22-2
587 suppliers
$5.00/10g